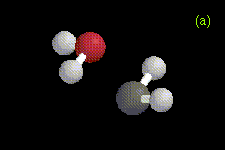
The optimized structures of the ylide-like complexes formed between singlet methylene and water or methanol at the MP2/6-31G* level, along with optimized structures for complexes formed between dimethylether and methylene at the RHF/3-21G level are shown in Figure 1. In these systems, the oxygen atom in water, methanol, or dimethylether is directed toward the carbene. The results obtained for the structure and energetics of the water-methylene complex are in agreement with previous theoretical work [1-3]. The geometries obtained for the complexes are very similar to those determined in an ab initio study of the interactions between singlet carbenes and fluoroalkanes [10].
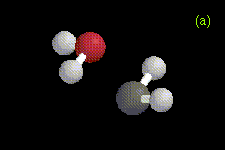
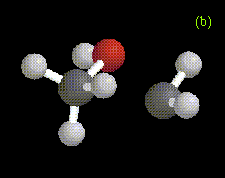
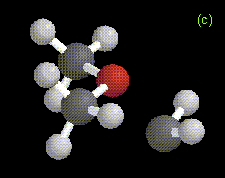
Figure 1. Optimized geometries at the MP2/6-31G* level for ylide-like complexes formed between (a) H2O and CH2, and (b) CH3OH and CH2. The distance from the oxygen to the carbene carbon is 1.81 Å in the H2O-CH2 complex, and 1.72 Å in the CH3OH-CH2 complex. Also shown is the optimized geometry at the RHF/3-21G level for the ylide-like complex formed between (c) CH3OCH3 and CH2. In this complex, the distance from the oxygen to the carbene carbon is 1.78 Å.
The optimized structure for the ylide-like complex formed between the allylic alcohol 2-methyl-3-buten-2-ol and methylene is shown in Figure 2a, along with the ylide formed between water and carbomethoxycarbene in Figure 2b. These structures were obtained at the RHF/3-21G level.
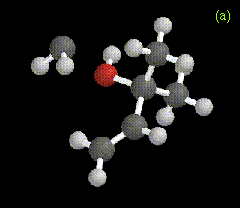
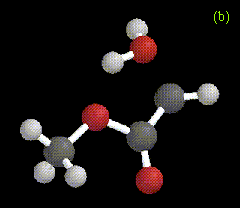
Figure 2. Optimized geometries at the MP2/6-31G* level for ylide-like complexes formed between (a) 2-methyl-3-buten-2-ol and CH2, and (b) H2O and CHCO2Me.
The binding energies for the complexes formed between water, methanol, or dimethylether and methylene, measured relative to the separated species, are listed in Table 1. The binding energies at the RHF/3-21G level are also listed for the 2-methyl-3-butene-2-ol complex with methylene, along with the water-carbomethoxycarbene complex. The binding energies for these ylide-like complexes range from 6 - 40 kcal/mol, depending on the basis set and level of theory. The CH3OH-CH2 complex is the most strongly bound complex involving methylene at all levels of theory employed so far, while the 2-methyl-3-butene-2-ol complex with methylene is the weakest. Note that the H2O-CHCO2Me complex is much stronger (by a factor of two) than the corresponding H2O-CH2 complex. This result is in agreement with a previous study of the interactions of carbenes with fluoroalkanes [10]. In the fluoroalkane-carbene systems, the binding energies of carbomethoxycarbene complexes were 1.3 - 1.7 times more strongly bound than the corresponding methylene complexes.
The binding energies determined at the RHF/3-21G level are within 2 kcal/mol of the results obtained at the highest level of theory (MP2/6-31G*). Ab initio studies on a wide variety of ylides [1-3] show that results for binding energies at the HF/3-21G level are similar to results obtained with larger basis sets and with the inclusion of electron correlation (due to cancellation of errors). Thus, we expect that for these systems, our RHF/3-21G results are qualitatively correct in their binding energy predictions.
To investigate the ylide-like nature of some of these carbene complexes, we have calculated the amount of electron transfer from the oxygen-containing species to the carbene. The results are summarized in Table 2. We have used Mulliken population analysis to obtain these values; however, natural population analysis yields similar results for the systems that we have looked at so far. The amount of electron transfer observed is similar for each of the complexes, approximately 0.2 at the HF/3-21G and MP2/6-3G* levels, and 0.1 at the HF/6-31G* level. For comparison, in ab initio studies of N-, O-, P-, and S-containing ylides [11], electron transfer was found to be the smallest for the O-containing ylides (0.2), and much larger for the N-, P- and S-containing ylides (0.4 - 0.5), indicating that the oxonium ylides are the weakest of the types studied.