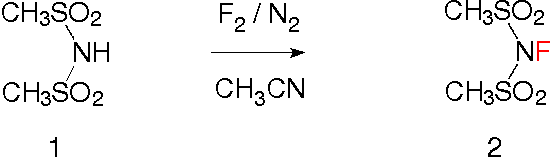
Email Discussion:
38 : N-Fluoro reagents
Nick Lawrence
Email Discussion:
38 : N-Fluoro reagents
Nick Lawrence
The preparation of the new reagent 2 is much less complicated than the synthesis of the analogous N-fluoro-trifluoromethanesulfonimide, which requires the reaction with elemental fluorine under pressure in a stainless steel bomb [4]. Starting from the simple imide 1, fluorination with 10% fluorine and 90% nitrogen in acetonitrile is sufficient.
The formation of cyclic N-fluoro-imide 4 from 3 is done under similar conditions as for the open N-fluoro-imide 2. The starting cyclic imide 3, however, is made by multistep synthesis.

An example for a reaction of the new reagent (2) is the fluorination of the deprotonated diethyl-2-phenyl-malonate (5) to give diethyl-2-fluoro-2-phenyl-malonate (6) together with some starting material. The speed of the fluorination with the new reagents is much slower compared to the speed with, e.g., 4-fluoro-1,4-diazoniabicyclo(2.2.2)octane salts [7]. One must also consider the possible deprotonation of the SO2-C-H groups in the new reagent by strong bases as a drawback under certain circumstances. In some cases this may be less important than better solubility, easy workup with less waste, and lower costs.
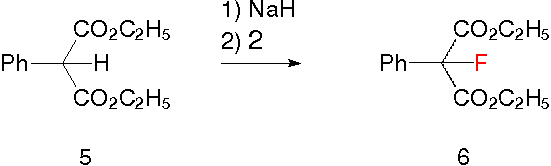
Further studies on properties and applications of the new fluorinating agents are in progress.
Into a solution of 15 g methanesulfonimide (1, Helferich and Flechsig, Chem. Ber. 75, p. 532, 1942) in 200 mL acetonitrile at -40 °C in the presence of 14.5 g sodium fluoride powder 60 L of fluorine and nitrogen gas (10 / 90, v/v) are bubbled during 2.5 h. After purging the reaction with pure nitrogen, the solvent is removed under reduced pressure, diluted with 200 mL ethyl acetate, filtered over Celite, concentrated under reduced pressure and recrystallised form hexane / ethyl acetate. One obtains 14.9 g N-fluoromethanesulfonimide (2) as pale yellow crystals melting at 45 - 48 °C. 1H-NMR (CDCl3): 3.42 ppm (d, J = 2 Hz, 6 H); Anal. Calcd for C2 H6 F N O4 S2 (191.21): C, 12.56%; H, 3.16 %; N, 7.33%. Found: C, 12.75%; H, 3.04%; N, 7.35%. CAS: [160974-18-5].
Into a solution of 370 mg [1,3,2]dithiazinane-1,1,3,3-tetraoxide (3, G. Geisler and R. Kuschmiers, Chem. Ber. 91, p. 1881, 1958) in 23 mL acetonitrile at -40 °C in the presence of 82 mg sodium fluoride powder 0.5 L of fluorine and nitrogen gas (10 / 90, v/v) are bubbled during 9 minutes. Afterwards, the reaction is purged by clean nitrogen, the solvent is removed under reduced pressure, dissolved in 10 mL dichloromethane, chromatographed on 60 g silica gel and recrystallised form hexane / acetone. One obtains 331 mg N-fluoro[1,3,2]dithiazinane-1,1,3,3-tetraoxide as crystals (4) melting at 171 - 172 °C. 1H-NMR (CD3CN): 2.47 ppm (m, 2 H), 3.72 ppm (m, 4 H) ; 19F-NMR (CD3CN): -34.4 ppm (ref. to CFCl3 = 0 and int. C6F6 = -162 ppm). Anal. Calcd for C3 H6 F N O4 S2 (203.22): C, 17.73%; H, 2.98 %; N, 6.89%. Found: C, 18.04%; H, 3.02%; N, 6.85%.
To a solution of 1.18 g diethyl-2-phenyl-malonate (5) in 10 mL dimethylformamide 200 mg sodium hydride (60% in oil) are added. This mixture is stirred for 1 h at 20 °C, then cooled to 0 °C and treated with 956 mg N-fluoromethanesulfonimide for 0.5 h. Then water is added, extracted with ether, washed with water, dried over sodiumsulfate and concentrated under reduced pressure. One obtains 1.353 g crude diethyl-2-fluoro-2-phenyl-malonate (6, lit.: 19F-NMR: -162.2 ppm W. E. Barnette, J. Am. Chem. Soc. 106, p. 452, 1984).
[1] J. A. Wilkinson: Recent advances in the selective formation
of the carbon-fluorine bond, Chem. Rev 1992, 92,
505-519.
[2] K. Sudlow, A. A. Woolf: Ordering of electrophilic fluorinating
reagents of the N-F class, J. Fluorine Chem. 1994,
66, 9-11.
[3] a) E. Differding, H. Ofner: N-fluorobenzenesulfonimide: a
practical reagent for electrophilic fluorinations, Synlett
1991, 187-9; b) E. Differding, R. O. Duthaler, A. Krieger,
G. M. Rueegg, C. Schmit: Electrophilic fluorinations with N-fluorobenzenesulfonimide:
convenient access to a-fluoro- and a, a-difluorophosphonates,
Synlett 1991, 395-6; c) E. Differding (Allied-Signal
Inc.): N-fluorosulfonimides and their application as electrophilic
fluorinating agents, US. 5254732, P: 6 pp., PAT APPL: 843692,
(28.02.92), Chem. Abstr. 120, 134027; d) W. J. Wagner,
G. A. Shia, A. J. Poss (Allied-Signal Inc.): Preparation of N-fluoro
sulfonimides from an alkali metal salt of a sulfonimide, PCT Int
Appl 94 08955, P: 18 pp., PRTY APPL: 963870 (US), (20.10.92).
PAT APPL: 93/US10059, (20.10.93), Chem. Abstr. 122,
191006.