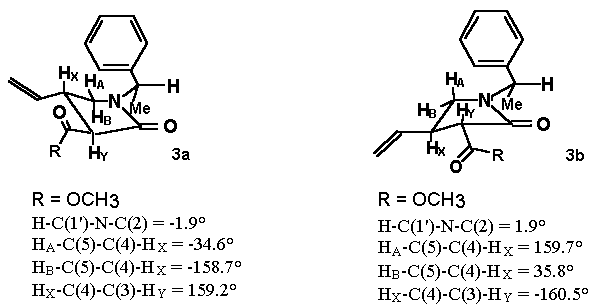
3a HA: 3.05 ppm; HB: 3.18 ppm; JAX: 9.5; JBX: 6.3
3b HA: 2.71 ppm; HB: 3.52 ppm; JAX: 7.5; JBX: 8.1
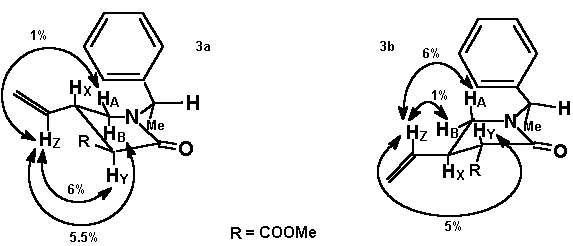
On irradiation at the vinyl proton HZ in the chain of the major isomer a large NOE enhancement of HB occurs, indicating the cis- relationship between HB and the substituent at C-4, whereas a small enhancement is observed for HA. The inverted trend is observed for the minor isomer in which a NOE effect is observed between HZ and HA. The configuration at C3 can be also assigned from NOE experiments.

The conformational analysis of the two diastereomers was performed using molecular mechanics calculations.
The force field used was MM+ and the geometry of the two structures was optimized using the Pollack-Ribierre algoritm.
The energy difference of 1.3 Kcal/mol shows that 3a is more stable than 3b.
The MM+ force field is an extension of MM2 which was developed by Allinger and co-workers. As references see: (a) Allinger, N.L., J. Am. Chem. Soc., 1977, 99, 8127. (b) Allinger, N.L., Yuh, Y.H., Quantum Chemistry Program Exchange, Bloomington, Indiana, Program #395, "Molecular Mechanics", Burkert, U.; Allinger, N.L., edd., ACS Monograph 177, American Chemical Society, Washington, D.C., 1982. (c) Lii, J.; Gallion, S.; Bender, C.; Wikstrom, H.; Allinger, N.L., Flurchick, K.M.; Teeter, M.M., J. Comp. Chem., 1989, 10, 503. (d) Lipkowitz, K.B., QCPE Bulletin, Indiana University, 12, 1 (Feb. 1992). The program is enclosed in Hyperchem package, available from Hypercube, Waterloo, Canada.
Minimum energy conformations and selected dihedral angles for 3a and 3b
.