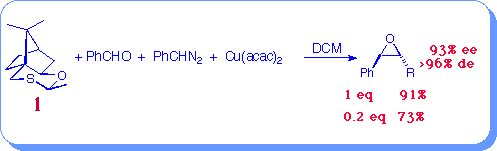
High yields and enantioselectivity in stilbene oxide formation was obtained using sulfide (1). Furthermore, no reduction in enantioselectivity was observed when using catalytic amounts of sulfide indicating that epoxide is derived solely from reaction of the sulfur ylide with the aldehyde.

The catalytic conditions were tested with a range of sulfides.
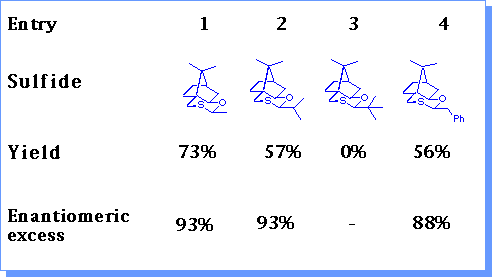
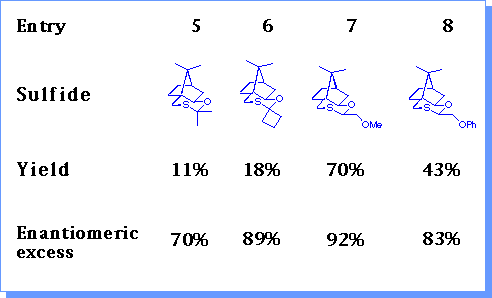
Increasing the size of the group a to sulfur resulted in reduced yields of epoxides (entries 1-3). Heteroatoms could be tolerated (entry 7) although the presence of an aromatic ring resulted in a reduction in enantioselectivity (entry 4, 8). The presence of two groups a to sulfur resulted in much reduced yields due to their increased steric hinderance (entry 5, 6). The optimum sulfide (1) was that derived from acetaldehyde .