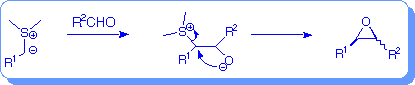
An alternative route to epoxides is the reaction of sulfur ylides with carbonyl groups. Sulfur ylide epoxidation is a C-C bond forming process in addition to being a C-O bond forming process.

To date the highest enantioselectivites have been obtained by Durst3 and Solladie-Cavallo4
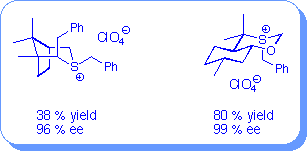
The results shown are for the preparation of trans-stilbene oxide using phase transfer conditons in which the ylide is generated in situ from the corresponding sulfonium salts in the presence of a base.