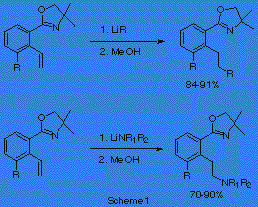
We have reported that alkyllithiums1 and lithium amides2 reacted with o-vinylphenyloxazolines to give addition products in good yields (scheme 1). It is also known that styrene suffers addition of alkyllithiums to the exocyclic double bond.3

Results:
Here, we studied the influence that the absence of the oxazoline group could have in the course of the reaction. We did our experiments with a carboxyl group instead and we observed lower yields than with the oxazoline group in the ring (scheme 2).
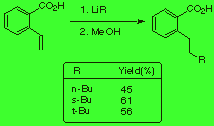
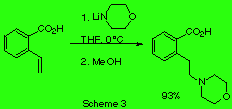
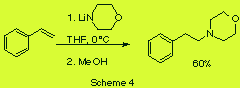
We studied as well reaction of lithium morpholide with o- carboxystyrene (scheme 3) and with styrene itself (scheme 4).
Conclusions:
Acknowledgments
Financial support form Spanish DGICYT (PB93-0647) is gratefully acknowledged.References: