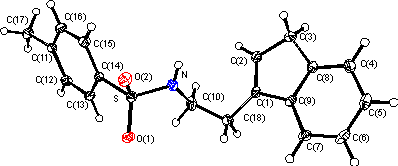

The structure shows this molecule to be a 1,2-disubstituted ethane with a regular indenyl moiety substituted at the 1 carbon of the five membered ring and having the double bond in the expected 1 position (C(1)...C(2) 1.34 Å) The sulfonamido moiety displays a planar nitrogen atom. There are no significant deviations from expected bond lengths and angles in the molecule. An active molecule (in .pdb format) is available here.
G.J. Gainsford, C. Lensink Acta Cryst. C, 1996, 52, 2.
Crystal data and structure refinement
Selected bond lengths and angles
Table 1. Crystal data and structure refinement for 4a.
Identification code [C455]shelxl
Empirical formula C18 H19 N O2 S
Formula weight 313.40
Temperature 130(2) K
Wavelength 0.71073 A
Crystal system Monoclinic
Space group P 2~1~/n
Unit cell dimensions a = 12.681(7) A alpha = 90 deg.
b = 7.261(4) A beta = 93.65(2) deg.
c = 16.654(9) A gamma = 90 deg.
Volume 1530.3(15) A^3
Z 4
Density (calculated) 1.360 Mg/m^3
Absorption coefficient 0.218 mm^-1
F(000) 664
Crystal size 0.82 x 0.18 x 0.08 mm
Theta range for data collection 2.08 to 24.98 deg.
Index ranges -15<=h<=2, 0<=k<=8, -19<=l<=19
Reflections collected 3216
independent reflections 2693 [r(int) = 0.0272]
absorption correction \y scan
max. and min. transmission 0.768 and 0.736
refinement method full-matrix least-squares on f^2
data / restraints / parameters 2693 / 0 / 204
goodness-of-fit on f^2 1.044
final r indices [i>2sigma(I)] R1 = 0.0454, wR2 = 0.0925
R indices (all data) R1 = 0.0731, wR2 = 0.1053
Extinction coefficient 0.0
Largest diff. peak and hole 0.219 and -0.330 e.A^-3
Table 2. Selected bond lengths [A] and angles [deg] for 4a.
_____________________________________________________________
S(1)-O(1) 1.431(2)
S(1)-O(2) 1.435(2)
S(1)-N(1) 1.600(3)
S(1)-C(14) 1.768(3)
N(1)-C(10) 1.467(4)
C(1)-C(2) 1.340(4)
C(1)-C(9) 1.475(3)
C(1)-C(18) 1.500(4)
C(2)-C(3) 1.493(4)
C(3)-C(8) 1.501(3)
C(4)-C(8) 1.380(4)
C(4)-C(5) 1.398(4)
C(5)-C(6) 1.381(4)
C(6)-C(7) 1.388(4)
C(7)-C(9) 1.389(4)
C(8)-C(9) 1.398(4)
C(10)-C(18) 1.530(4)
C(11)-C(16) 1.390(4)
C(11)-C(12) 1.394(4)
C(11)-C(17) 1.506(3)
C(12)-C(13) 1.388(3)
C(13)-C(14) 1.389(3)
C(14)-C(15) 1.389(3)
C(15)-C(16) 1.377(4)
O(1)-S(1)-O(2) 119.49(12)
O(1)-S(1)-N(1) 107.40(13)
O(2)-S(1)-N(1) 106.52(12)
O(1)-S(1)-C(14) 107.11(11)
O(2)-S(1)-C(14) 106.77(11)
N(1)-S(1)-C(14) 109.30(13)
C(10)-N(1)-S(1) 123.5(2)
C(2)-C(1)-C(9) 108.0(2)
C(2)-C(1)-C(18) 128.2(2)
C(9)-C(1)-C(18) 123.8(2)
C(1)-C(2)-C(3) 111.9(2)
C(2)-C(3)-C(8) 102.7(2)
C(8)-C(4)-C(5) 118.6(3)
C(6)-C(5)-C(4) 120.5(2)
C(5)-C(6)-C(7) 121.1(3)
C(6)-C(7)-C(9) 118.6(3)
C(4)-C(8)-C(9) 120.9(2)
C(4)-C(8)-C(3) 130.6(2)
C(9)-C(8)-C(3) 108.5(2)
C(7)-C(9)-C(8) 120.3(2)
C(7)-C(9)-C(1) 131.0(2)
C(8)-C(9)-C(1) 108.7(2)
N(1)-C(10)-C(18) 111.0(2)
C(16)-C(11)-C(12) 118.3(2)
C(16)-C(11)-C(17) 120.5(2)
C(12)-C(11)-C(17) 121.2(2)
C(13)-C(12)-C(11) 121.2(2)
C(12)-C(13)-C(14) 119.2(2)
C(15)-C(14)-C(13) 120.4(2)
C(15)-C(14)-S(1) 119.9(2)
C(13)-C(14)-S(1) 119.7(2)
C(16)-C(15)-C(14) 119.6(2)
C(15)-C(16)-C(11) 121.4(2)
C(1)-C(18)-C(10) 113.3(2)