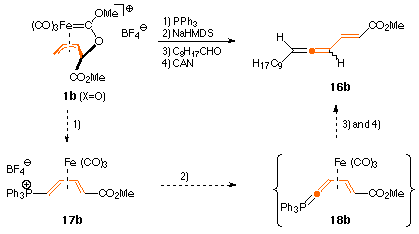

Like enolates, phosphanes attack allyl-ironcarbene complexes 1 at the allylic terminus C-6 to give air-stable (3E)-4-phosphonio-1,3-diene tricarbonyliron complexes 17. These can be deprotonated at -78 °C with strong bases like sodium hexamethyldisilylamide (NaHMDS) at the 4-position to give the corresponding highly reactive cumulated ylids 18 as deep red solutions in THF. Complexes 18 are not isolable but undergo Wittig olefination reactions with aldehydes furnishing - after demetalation with CAN - vinylallenes (i.e. 1,2,4 -trienes) 16 in moderate to good yields.
The entire sequence can be performed in one pot as is depicted above for the synthesis of the pheromone 16b of the male dried-bean weevil acanthoscelides obtectus in ca. 45% overall yield.