| [Related articles/posters: 041 028 009 ] |
We have previously reported the stereocontrolled addition of both 2-lithiothiazole and 2-lithiofuran to the differentially protected nitrones 1 and 2 derived from L-serine[3a,b]. The high stereocontrol observed in those reactions, explained on the basis of the different protecting groups arrangement, prompted us to extend our investigation to nitrones 3 and 4, derived from L-threonine.
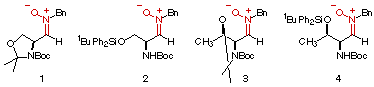
In this communication we report our findings about the addition of 2-lithiothiazole and 2-lithiofuran to nitrones 3 and 4. Also, a mechanistical explanation based on semiempirical calculations is given in order to rationalize the different behaviour of nitrones 3 and 4 when they are compared with nitrones 1 and 2, respectively
The organometallic reagents (2-lithiofuran and 2-lithiothiazole) were generated in situ as described[4] and then added under an inert atmosphere to a cooled (-80oC) solution of the corresponding nitrone. An excess of 3.0 equivalents of organometallic derivative was used in all cases, the use of stoichiometric amounts of organometallic reagent giving rise to recovering of c.a. 50% of starting material. The reaction proceeded smoothly to give a mixture of hydroxylamines after quenching with saturated aqueous ammonium chloride and extraction with diethyl ether (Scheme). The diastereomeric hydroxylamines were easily separated by column chromatography.
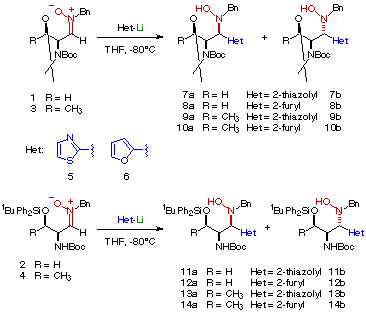
As evidenced in Table 1, whereas a high stereocontrol was observed with nitrones 1 and 2, the syn adducts were obtained as the major products in the additions to nitrones 3 and 4. In the case of N,N-diprotected nitrones 1 and 3 only one isomer could be detected by 1H-NMR spectroscopy after the addition reaction.
| nitrone
|
Het-Li
|
syn
: anti
|
hydroxylamine
|
yield
|
| 1
|
5
|
>95 : 5
|
7
|
89
|
| 1
|
6
|
>95 : 5
|
8
|
92
|
| 2
|
5
|
12 : 88
|
11
|
87
|
| 2
|
6
|
10 : 90
|
12
|
90
|
| 3
|
5
|
>95 : 5
|
9
|
86
|
| 3
|
6
|
>95 : 5
|
10
|
91
|
| 4
|
5
|
85 : 15
|
13
|
90
|
| 4
|
6
|
86 : 14
|
14
|
87
|
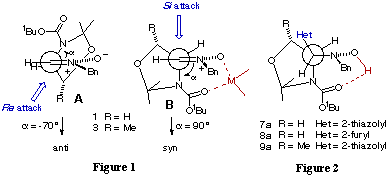
In order to assess the various factors contributing to the quite different behaviour of nitrones derived from L-serine and L-threonine we have carried semiempirical energy calculations[7] (MOPAC 6.0)[8] using AM1 methods[9]. Both nitrones 1 and 3 showed similar results. So, it is possibe to conclude that the methyl group has a little effect on the stereoselectivity of the addition to nitrones 1 and 3. Among the 37 conformers considered for nitrones 1 and 3 in ground state the most stable was found to be conformer A with a dihedral angle of -70o (Figure 1). However the fact that an excess of organometallic reagent is needed to achieve a high degree of conversion in the nucleophilic addition reflects some sort of chelation control in the transition state. Consistent to the high degrees of syn selectivity observed, conformer B (Houk model[10]) can be invoked. This conformer is only 1.0 Kcal/mol higher in energy than the most stable conformer A. In agreement with this hypothesis is the observation that the X-ray structures of hydroxylamines 7a, 8a and 9a present intramolecular hydrogen interactions as shown in Figure 2 [3c],[11]. On the basis of the assumption that the reaction proceeds via a product-like transition state, conformer B (Figure 1) appears as the more plausible model.
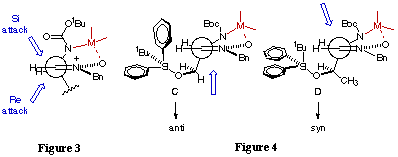
An explanation of the different behaviour of nitrones 2 and 4 upon the addition of 2-lithiothiazole and 2-lithiofuran needs of a more detailed study. In fact, the spatial orientation of the tert-butyldiphenylsiloxy group is crucial for the attack of the organometallic reagent. In the case of the nitrone 2 the energy minimum corresponds to a conformer with a dihedral angle of -16.1o in which the tert-butyldiphenylsiloxy group is oriented in such a way that the Si face is clearly more hindered than the Re face (Figure 4, conformer C). The energy minimum for nitrone 4 corresponds to conformation represented by D (Figure 4) in which the dihedral angle is -0.78o In this conformation the presence of the methyl group completely hinders the entry of the nucleophile by the Re face. In addition the slightly different disposition of the tertbutyldiphenylsiloxy group makes the only accessible Si face less hindered that in the case of nitrone 3, thus explaining the observed opposite selectivity.
Admittedly, this proposal only indicates a part of the possible reaction courses for the studied reactions. Further consideration for the stereochemistry of the addition reactions to nitrones requires more ellaborate calculations concerning both the transition states of the different nucleophilic additions and stereochemical models in which the nitrone are associated to a metal atom. These investigations are currently underway in our laboratories and they will be reported in due course.
[1] (a) Reetz, M.T.; Reif, W.; Holdgrun, X. Heterocycles 1989, 28, 707-710. (b) Jurczack, J.; Pikul, S.; Bauer, T. Tetrahedron 1986, 42, 446-488. (c) Yokoyama, M.; Toyoshima, H.; Shimizu, M.; Togo, H. J. Chem. Soc. Perkin Trans i 1997, 29-33. (d) Hodges, J.C.; Patt, W.C.; Connolly, c.J. J. Org. Chem. 1991, 56, 449-452. (e) Class, Y.J.; DeShong, P. Tetrahedron Lett. 1995, 36, 7631-7634. (f) Martin, S.F.; Chen, H.-J.; Lynch, V.M J. Org. Chem. 1995, 60, 276-278. (g) Dondoni, A.; Merino, P. Thiazoles In Comprehensive Organic Chemistry, 2nd Edition, Vol. 3. Cap. 6. Eds. A.R. Katritzky, C.W. Rees, E.F.V. Scriven, Elsevier. 1996, and references cited therein. (h) Albright, J.D.; Howell, C.F.; Sum, F.W. Heterocycles 1993, 35, 737-754. (i) Szechner, B. Tetrahedron 1981, 37, 949-952. (j) Mukaiyama, T.; Tsuzuki, R.; Kato, J.I. Chem. Lett. 1985, 837-840. (k) Pikul, S.; Raczko, J.; Ankner, K.; Jurczack, J. J. Am. Chem. Soc. 1987, 109, 3981-3987. (l) Mukaiyama, T.; Suzuki, K.; Yamada, T.; Tabusa, F. Tetrahedron 1990, 46, 265-276.
[2] (a) Risch, N.; Arend, M. Formation of C-C Bonds by Addition to Imino Groups (C=N) In Stereoselective Synthesis Helmchen, G.; Hoffmann, R.W.; Mulzer, J.; Schaumann, E. (Eds), Houben-Weyl, Thieme, Stuttgart, 1996, Vol. 3, pp. 1833-1929. (b) Horenstein, B.A.; Zabinski, R.F.; Schramm, V.L. Tetrahedron Lett. 1993, 34, 7213-7216. (c) Basha, A.; Henry, R.; McLaughlin, M.A.; Ratajczyk, J.D.; Wittenberger, S.J. J. Org. Chem. 1994, 59, 6103-6106.
[3] (a) Lanaspa, A.; Merchan, F.L.; Merino, P.; Tejero, T.; Dondoni, A. Electronic Conference on Trends in Organic Chemistry (ECTOC-1) Rzepa, H.S.; Goodman, J.G. (Eds.), CD-ROM, Royal Society of Chemistry Publications, 1995. (b) Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T.; Bertolasi, V. Chem. Eur. J. 1995, 1, 505-520. (b) Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Synthesis 1994, 1450-1456. (c) Dondoni, A.; Merchan, F.L.; Merino, P.; Tejero, T. Chem. Commun. 1994, 1731-1733.
[4] Brandsma, L.; Verkruijsse, H. Preparative Polar Organometallic Chemistry Springer-Verlag, Berlin, 1987. For preparation of 2-lithiothiazole see: vol. 1, pp. 138-139. For preparation of 2-lithiofuran see: vol 1, pp. 126-129.
[5] Merino, P.; Merchan, F.L.; Tejero, T.; Lanaspa, A. Acta Cryst. 1996, 52, 2536-2538.
[6] Tejero, T.; Franco, S.; Junquera, F.; Lanaspa, A.; Merchan, F.L.; Merino, P.; Rojo, I. Tetrahedron: Asymm. 1996, 7, 1529-1534.
[7] In order to simplify calculations tert-butyl and benzyl groups have been replaced by methyl groups.
[8] Stewart, J.J.P. QCPE 1990, 445.
[9] Dewar, M.J.S.; Zoebisch, E.G.; Healy, E.F.; Stewart, J.J.P. J. Am. Chem. Soc. 1985, 107, 3902-3909.
[10] Houk, K.N.; Paddon-Row, M.N.; Rondan, N.G.; Wu, Y.-D.; Brown, F.K.; Spellmeyer, D.C.; Metz, J.T.; Li, Y.; Loncharich, R.J. Science 1986, 231, 1108-1117.
[11] Merino, P.; Merchan, F.L.; Tejero, T.; Lanaspa, A. Acta Cryst. Sect. C 1996, 52, 2536-2538.
[12] Merino, P.; Merchan, F.L.; Tejero, T.; Lanaspa, A. Acta Cryst. Sect. C 1995, 51, 1949-1950.
[13] Merino, P.; Merchan, F.L.; Tejero,T.; Lanaspa, A. Acta Cryst. Sect. C 1996, 52, 218-219.