Current Research
Within the subclass of the Gly-Gly model, we decided to examine
the effect that C(2)-C(6) Aca linker monoalkylation has on the product
macrocycle conformation. Specifically, we chose to synthesize the
benzyl substituted linker derivatives to alleviate solubility problems
which previously hindered analysis of the related methylated substrates.
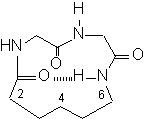
These cyclic tripeptides are now being subjected to NMR,
CD, and X-ray crystallograpic analysis to determine their type and/or bias
for type I/II b-turn conformations. This
poster describes the synthetic approaches toward the macrocycles and some
preliminary results from X-ray crytallographic analysis.
Back to Index
Synthesis of (8S)-Benzyl-1,4,7-triazacyclotridecan-3,6,13-trione

