1.1. Importance of the "pigments of life"
The studies of the tetrapyrrolic dyes, which have adequately been called the "pigments of life"[1,2] has attracted the attention of chemists and biologists alike.[3-5] The "pigments of life" play an important role as cofactors for processes like photosynthesis,[6] oxygen transport,[7], oxidation processes,[8,9] methane synthesis[10-13] and for a series of unusual rearrangements.[14,15] Experimental studies of the tetrapyrrole biosynthesis started with Shemin's seminal experiments[16] proving the incorporation of glycine into the pigments of the red blood cells.[17] In an impressive series of investigations the major steps leading from 5-aminolevulinic acid to the skeleton of all tetrapyrroles were characterised.[18-22] The structures of the most important intermediates were determined.[23,24] In a very short period the central part of the biosynthesis, namely the sequence leading from the dedicated, unusual amino acid 5-aminolevulinic acid via the pyrrole derivative porphobilinogen (=PBG) to the first tetrapyrrolic intermediate, uroporphyrinogen III was established (Scheme 1).
Scheme 1: Central part of the biosynthesis of the "pigments of life"
1.2. Relation between the biosynthetic pathway and a potential prebiotic pathway to porphobilinogen
The central part of the biosynthesis creates in only three enzyme catalysed steps the tetrapyrrolic core common to all "pigments of life" (Scheme 1). This convergent and highly efficient part of the biosynthesis has been maintained in all living organisms. This observation has been taken as a strong indication that these biosynthetic steps have been preserved during the whole process of evolution.[25-27] For the biosynthesis of the first dedicated precursor the 5-aminolevulinic acid two distinct pathways are known today.[28-31] Starting from uroporphyrinogen III different pathways lead to the various, distinct naturally occurring tetrapyrrolic pigments.[32-34] In same cases two separate pathways have been developed in different organisms for the same molecule, as shown recently for the biosynthesis of vitamin B12.[35,36] In view of this fact it is all the more surprising that the central part of the biosynthetic pathway has been maintained so rigorously. This lead to the hypothesis that the "pigments of life" have been formed already during the prebiotic period.[26,27,37-43] Two of the three enzymatic steps forming the tetrapyrrolic skeleton can be simulated chemically in the absence of any enzyme.[44-47]
However the deceivingly simple Knorr condensation transforming 5-aminolevulinate into porphobilinogen could not be imitated chemically so far. There is a dichotomy between the chemical reactivity of 5-aminolevulinate and the biosynthesis leading to porphobilinogen. 5-Aminolevulinate dimerizes chemically to form a dihydropyrazine via two successive C-N bond forming steps (Scheme 2).[48] The oxidised form the pyrazine can be isolated in good yield, whereas pyrroles are only minor components of the chemical dimerization process.
Scheme 2: Chemical dimerization of 5-aminolevulinate.[48]
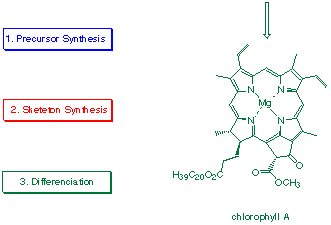
A systematic search for potential prebiotic pathways to porphobilinogen or prebiotic equivalents of porphobilinogen was undertaken in the laboratories of Eschenmoser.[38,40] Under prebiotic boundary conditions a synthesis of the "cyano-equivalent" of desaminomethyl-porphobilinogen could be achieved.[38,49] The proposed prebiotic pathway is sufficiently far away from today's biosynthesis that no mutual reinforcing argument has emerged from these studies (see Scheme 3). One of the major difficulties is clearly that the mechanism for the biochemical porphobilinogen formation is not known. We are therefore unable to recreate the sequences of events leading to the monopyrrolic building block. We do not know if part or all of the prebiotic pathway has been maintained in today's biosynthesis, because we do not understand the details of the biosynthesis itself.
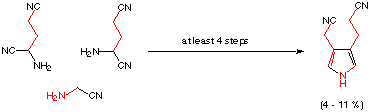
Scheme 3: Proposed prebiotic synthesis of a desaminomethyl-porphobilinogen analogue [38]
1.3. Mechanistic alternatives for the biosynthesis of porphobilinogen in comparison with a recent synthesis
During the biosynthetic dimerization of 5-aminolevulinate a C-C-bond forming process and a C-N-bond forming process have to occur in a sequence controlled by the enzyme. Both possible sequences of events have been proposed for the mechanism of porphobilinogen synthase (PBGS = EC 4.2.1.24) the second dedicated enzyme on the pathway to the "pigments of life".[50-52] No firm proof has been brought forward so far for either of the mechanisms (Scheme 4).[53]
Scheme 4: The two mechanistic proposals for the biosynthesis of porphobilinogen [50,51]
We could recently show, that the aldol product, which unites the two 5-aminolevulinate units in the asymmetric way necessary for the PBG synthesis is transformed without the help of any protein into the product, the porphobilinogen (see Scheme 5).[54] This observation allows to attribute a chemical logic to a mechanism where the rate determining C-C bond forming step is executed first.[53,54] Studying the mechanism of PBGS whose transformation could not be chemically imitated so far, should give us relevant information on the way enzymes solve such difficult synthetic problems.
Scheme 5: The chemical synthesis of porphobilinogen modelled according to the mechanistic proposal of Shemin [54]
1.4. Biochemical knowledge about the enzyme porphobilinogen synthase
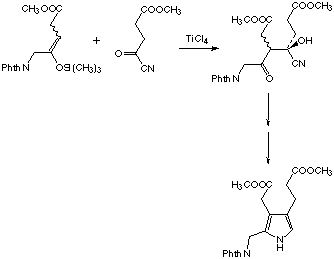
Until the advent of the methods of genetic engineering the studies of porphobilinogen synthase were hampered by the relatively small amounts of enzyme available. Despite the problem with the availability of the enzyme some important information could be obtained during this period (Scheme 6). Shemin was the first to show that the e-amino group of a lysine at the active site forms a Schiff base with the ketogroup of the substrate.[50] Stereospecific labelling of one of the protons at C5 of 5-aminolevulinic acid allowed to prove that the deprotonation at the position C5 is stereoselective and must therefore still be under the control of the enzyme.[55,56] Jordan's beautiful pulse labelling experiment proved, for the enzymes tested, that the first substrate to form a Michaelis complex with the enzyme was the substrate which is incorporated at the "P-side" of porphobilinogen synthase.[51] Finally Jaffe could show with the help of very challenging NMR-experiments that the Schiff base is probably present in the imine form and that the product PBG stays relatively tightly bound to enzyme.[57,58]
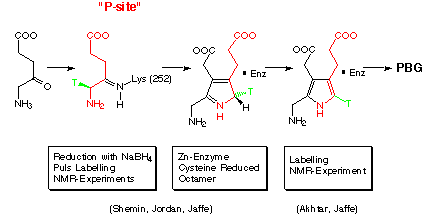
Scheme 6: Experimental datas concerning the mechanism of PBGS
1.5. Additional information about porphobilinogen synthase obtained with the help of genetic engineering
Recent years has seen a renewal of the interest in the biosynthetic pathways to the tetrapyrroles in general and of the step forming porphobilinogen in particular. The genes and thereby also the gene derived protein sequences from over 20 organisms are known.[59,60] This made large quantities of enzymes from different sources available. Despite this effort there has been no high resolution X-ray structure published so far.[61,62] In recent years the influence of different metal atoms on the efficiency of the enzyme was studied systematically.[63] It could be shown that divalent metal ions like Zn2+ and Mg2+ are playing crucial roles for the activity of the enzyme.[64] Detailed models for the role of the different metal ions have been proposed and a general classification for the enzymes from different sources was presented.[65,66] The active site of enzymes from different sources were modified by irreversible inhibitors and the resulting proteins studied by NMR-spectroscopy and by degradation.[67,68]
1.6. Recent inhibition studies of porphobilinogen synthase
In the absence of the definitive structural information on PBGS systematic inhibition studies were started.[69,70] These inhibition studies allowed to define the importance of the different parts of the structure of the substrate for recognition at the active site of the enzyme (Scheme 7).
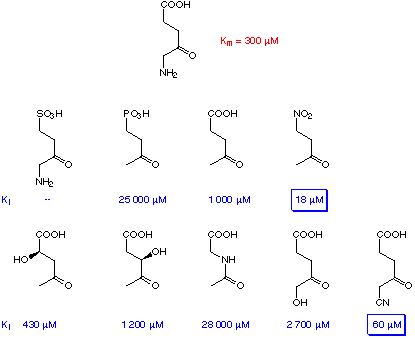
Scheme 7: Results of the inhibition tests of PBGS from Rhodopseudobacter spheroides.[70]
More recently the use of substrate analogues like the ester or thioester which could be irreversibly bound to the enzyme was reported (Scheme 8).[71-73]
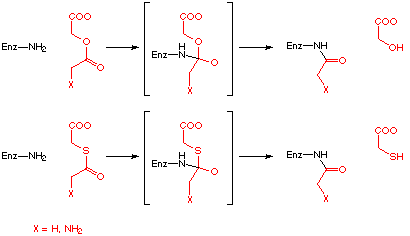
Scheme 8: Irreversible inhibition of PBGS as observed by Scott and Leeper.[71,73]
The enzyme-inhibitor complex could be identified with the help of electro-spray MS [73,74] and with 13C-NMR.[71] It has to be noted that in one case the concentration of the inhibitor needed to obtain irreversible binding to the enzyme has to be higher than the inhibition constants determined kinetically.[71] This observation is analogous to the results obtained with the 5-chlorolevulinate.[67,75] For the 5-chlorolevulinate the discrepancy between the kinetic results and the experiments intended to prove the irreversible inhibition could have been due to the partial hydrolysis of the 5-chlorolevulinate to the 5-hydroxylevulinate under the incubation conditions.[76] This explanation can not be applied to the ester and the thioester derivative. Recently the results of a systematic search for inhibitors and the testing of these compounds with PBGS from Bacillus subtilis has been reported by the group of Leeper.[73]
1.7. Structure of inhibitors which could be used in biased libraries
We report here on our efforts to synthesise specifically designed inhibitors for PBGS. The inhibitors should mimic the structures of the postulated intermediates. The results of the inhibition studies should allow us to follow the mechanism of the enzyme catalysed pathway. On top of these kinetic studies the inhibitors will be very useful as soon as the first X-ray structure of PBGS will become available. Structures of enzyme inhibitor complexes will allow to see the relevant interactions between the active site and the intermediates and therefore allow to gain a deeper insight into the mechanism of this enzyme.
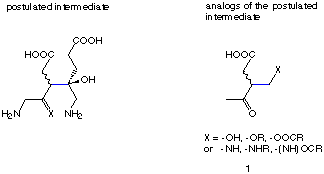
Scheme 9: Analogues of the intermediate postulated by Shemin
We decided to study synthetic avenues which should allow to create quickly not only one derivative but series of similar derivatives of the potential intermediate of the Shemin mechanism (see Scheme 9).[77-79] We consider the formation of the C-C-bond between the two identical substrates as the central step of this mechanism.[53,54] As a consequence of the results of his pulse-labelling experiments Jordan has postulated two mechanisms.[80] In both his mechanisms the first substrate is going to the "P-site" of the product and it is this substrate which is bound by a Schiff base to the enzyme (Scheme 4 and 6).[51,52] In the second mechanism the same C-C-bond is formed first as it has been postulated by Shemin.[81,82] Therefore structural analogues as we propose them could be good inhibitors if the initial Shemin mechanism or if the second mechanism postulated by Jordan is working.[83,84]
The synthesis of the proposed structural analogues of the postulated intermediates should allow to introduce a wide variety of different substituents at the position X. This approach should permit to optimise the interaction of the inhibitors with the active site of the enzyme. If compounds of the type 1 can be obtained in sufficient quantities even the synthesis of biased libraries can be envisaged.[85] The goal of these studies is to obtain optimal inhibitors of the structural class we propose. Having in hand efficient inhibitors allows to analyse the factors responsible for the interaction between the inhibitor molecules and the active site. More importantly enzymes from different sources can then be compared and the adaptation of PBGS during the process of evolution can be detected.