![]()
![]()
3. Aza-Claisen rearrangement with N-allyl-N-benzylketene-N,O-silyl acetal
3.1. Synthesis of N-allyl-N-benzylketene-N,O-silyl acetal (11)
To verify the behavior of the tandem intermediates towards Lewis acid we decided to synthesize the compounds 10a,b (scheme 11):
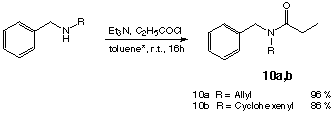
scheme 11
The amides 10a,b have been synthesized without problems using the method of Habisch [39] (scheme 11). The N,O-silyl acetals 11a,b were less stable than the N,O-silyl acetals 3a-e (scheme 5). Using the method already developed in our group [3,4] only products with unsatisfactory purity were obtained. Modification of this method lead to no product formation at all. In the case of cyclohexenyl amide 10b no N,O-ketene acetal was detected. Because of the instability of 10a it was not possible to purify the products by distillation or flash-chromatography Therefore the yields of the reaction can not be determined precisely. Extraction of the raw reaction mixture against water, drying the pentane phase and than evaporation the solvent gave the raw product which was used directly. The quality of the raw material was checked with 1H-NMR. The purity of the product obtained was by far not satisfactory. We decided to use the raw product anyway to check the reactivity of the N,O-silyl ketene acetals.

scheme 12
![]()
3.2. Thermal and catalyzed rearrangement of 11
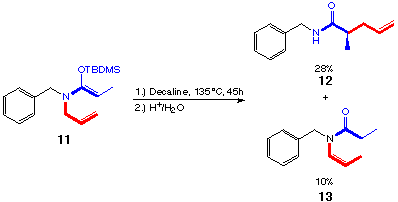
scheme 13
Starting with the unpurified ketene acetal 11a it was possible to isolate 28% of the rearrangement product 12 (scheme 13). Surprisingly also an isomerization product 13 of the hydrolyzed starting material could be isolated. As the purity of the starting material was not sufficient, the 28% of rearranged product is encouraging. In comparison with the ketene acetals with a benzoyl group 3a the rearrangement seems to work better in this case.
Nevertheless it was impossible to catalyze the rearrangement in this case, neither with Lewis acids nor with palladium(II).
![]()