Coumarins are nowadays an important group of organic compounds that are used as additives to food and cosmetics [1], optical brightening agents [2], and dispersed fluorescent and laser dyes [3]. The derivatives of coumarin usually occur as secondary metabolites present in seeds, root, and leaves of many plant species. Their function is far from clear, though suggestions include waste products, plant growth regulators, fungistats and bacteriostats [4]. It is therefore of utmost importance that the synthesis of coumarin and its derivatives should be achieved by a simple and effective method.
Coumarins can be synthesised by one of such methods as the Claisen rearrangement, Perkin reaction, Pechmann reaction as well as the Knoevenagel condensation [5]. Some of the industrially important coumarins are the 4-methylsubstituted group (e.g., 7-hydroxy-4-methylcoumarin (Coumarin 47 or Coumarin 460) and 7-diethylamino-4-methylcoumarin (Umbelliferon 47)). They can be prepared by the Pechman reaction, which is just suited to their preparation from readily available 1,3-disubstituted compounds and their acetoacetic esters .
It was recently shown that the Pechman reaction could be quickly achieved using microwave irradiation of the reagents in household microwave oven [6]. Since the solvent free phase-transfer catalytic reactions under microwave irradiation is the main topic in our lab [7], it has prompted us to present our results of the synthesis of coumarins by the Knoevenagel condensation under such conditions. However, previously both the Knoevenagel reaction [8] and synthesis of coumarin by the Knoevenagel condensation [9] have been the subject of microwave induced reactions, in the case of coumarins the only example that has been always given is the synthesis of 3-ethoxycarbonylcoumarin (i.e., ethyl 2H-1-benzopyran-2-oxo-3-carboxylate).
Results and Discussion
[first page]
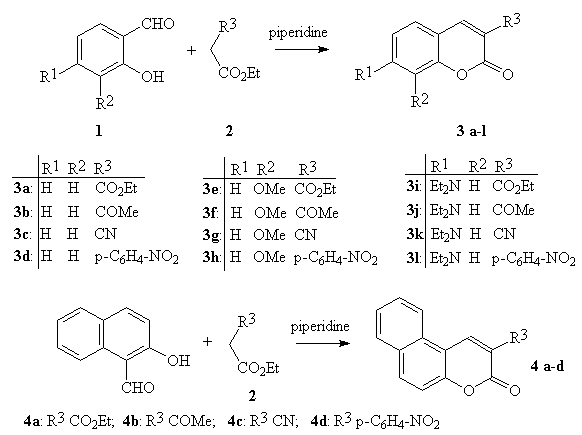
The aim of the present paper is to show that under the microwave irradiation the Knoevenagel condensation can be successfully applied to the synthesis of a number of coumarins, and the scope of the method is much broader. It is reported a very simple, fast and general procedure where the condensation of salicylaldehyde or its derivative with various derivatives of ethyl acetate (e.g., R3CH2COEt; R3: CO2Et, COMe, CN, p-C6H4-NO2) in the presence of piperidine under the solvent-free condition leads to coumarin synthesis (Fig.1)

Figure 1: Synthesis of coumarins synthesis by the Knoevenagel condensation under microwave irradiation.
The solvent-free conditions under microwave irradiation offers several advantages [10]: solvents are often expensive, toxic, difficult to remove in the case of aprotic dipolar solvents with high boiling point, and are environment polluting agents. Moreover, liquid-liquid extraction is avoided for the isolation of reaction products, and the absence of solvent prevents from the risk of hazardous explosions when the reaction takes place in a microwave oven. The reactions (i.e., the synthesis of coumarins) were usually completed within 1-10 min. and gave improvement yield over conventional method in a shorter time. Moreover, the work-up procedure is simply reduced to the recrystallization of product from an appropriate solvent. The results from the experiments are shown in Table 1.
TABLE 1: Results of the coumarins synthesis by the Knoevenagel reactions under microwave irradiation.
| Compound | Power [%] | Time [s] | Temp.a [C] | Yield [%] | M.p.e [C] |
| 3a b | 10 | 10 | 129 | 89 | 91-2 |
| 3b b | 20 | 1 | 90 | 94 | 120-2 |
| 3c b | 20 | 4 | 201 | 76 | 182-4 |
| 3d b | 40 | 5 | 220 | 85 | 274-5 |
| 3e b | 10 | 10 | 131 | 72 | 89-91 |
| 3f b | - | - | r.t.d | 90 | 167-9 |
| 3g b | - | - | r.t.d | 90 | 224-5 |
| 3h c | 10 | 5 | 90 | 78 | 294-6 |
| 3i b | 20 | 6 | 220 | 55 | 80-2 |
| 3j b | 20 | 6 | 136 | 88 | 152-3 |
| 3k b | 10 | 10 | 165 | 80 | 225-6 |
| 3l c | 10 | 6 | 122 | 90 | 265-7 |
| 4a b | 20 | 5 | 170 | 80 | 117-8 |
| 4b b | 10 | 8 | 87 | 75 | 186-8 |
| 4c b | 10 | 10 | 100 | 82 | 296-8 |
| 4d c | 20 | 3 | 170 | 75 | 297-9 |
In summary, the method describes a noticeable improvement in reactions conditions for the coumarin synthesis by the Knoevenagel condensation and takes advantage of both solvent free conditions reaction and microwave activation. As it is shown in Table 1, the reaction time is reduced to only a few minutes by using microwave dielectric heating. The reactions can be run safely in good yields, and the work-up procedure is reduced to the recrystallization of desired products.
The reactions were carried out under atmospheric pressure in an open vessel adapted to Synthwave 402 microwave monomode reactor (Prolabo). All the compounds were identified by GC/MS, IR, NMR and gave satisfactory results in comparison with authentic samples. Melting points are in good agreement with literature data.
General Procedure - A mixture of a hydroxyaldehyde (100 mmol), carbonyl compound 2 (110 mmol), and piperidine (0.20 g, 2.4 mmol) was irradiated and heated the microwave reactor with power and by the time indicated in Table 1. At the end of exposure to microwave, the reaction mixture was cooled to room temperature, and the crude product was recrystallised from an appropriate solvent (Table 1) to effort the coumarin 3 or 4.