R = Me, Et, i - Pr, Ph

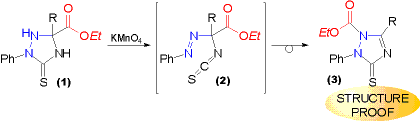
| When ethoxycarbonyl substituted triazolidine-3-thiones (1) are subjected to the permanganate oxidation only a transient spot characteristic for gem. azoisothiocyanates (2) can be detected. But only the rearranged product - the ethoxycarbonyl substituted triazoline-3-thione (3) can be isolated. | Two questions concerning the EXACT STRUCTURE AND ITS PROOF of the oxidation products arose from this reaction. The first one is concerned with the migrating group, the second one is concerned with the destination of the migrating group. |

| The question, which of the substituents migrated could be solved by the alkaline hydrolysis of the oxidation product (1) furnishing the known triazolinethione (2) unsubstituted in ring position 1. | The question about the position to which the ethoxycarbonyl group migrated was elucidated by an X-ray analysis on (1, R = Et), confirming the structure (1) shown. |
| TOPICS | G) OXIDATION ETHOXYCARBONYLTRIAZOLIDINETHIONES: |
| G1) General Reactivity G1.1) Structure Proof HOME INDEX |