The development of new methodologies enabling the synthesis of unnatural nucleosides plays a significant role in the search for new antitumor and antiviral agents. Following the discovery of AZT, DDC, DDI and d4T, as potent antiviral agents,1 acting as competitive inhibitors of the viral reverse transcriptase, the preparation of modified nucleosides with structural alterations in either the heterocyclic ring or the sugar moiety or both has become a very active research area and new synthetic methods have been designed and developed.2
Saturated and unsaturated 2',3'-dideoxynucleosides, containing different functionalities and lacking the 3'-hydroxyl group, are expected to terminate viral DNA synthesis after their incorporation in the chains. In this context, recently, a number of synthetic efforts have been focused on this type of nucleoside analogs.3 They are largely classified into two approaches. The first is based on the structural modification of the sugar part of intact nucleosides available from natural sources, the second one consists of the coupling of suitably modified sugars with nucleoside bases, eventually followed by formation of a double bond at the 2',3'-position.
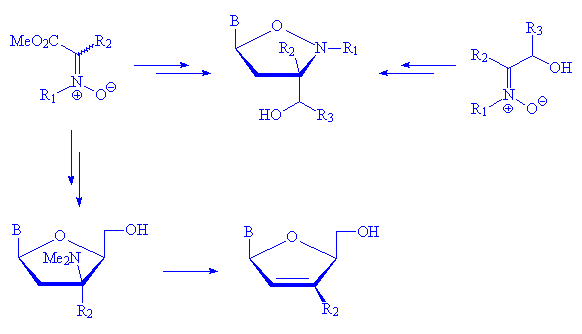
Accordingly, compounds containing an unsaturated sugar moiety and isoxazolidine nucleosides with N-alkyl- or hydroxymethyl groups have been reported.4
On this bases, we wish report here our recent results that, starting from
variously substituted C-alkoxycarbonyl nitrones,5 allow
for the construction of N,O-nucleosides, 2',3'-dideoxynucleosides,
d4T and its 2'-methyl analogs.
References
- E. De Clerq, J. Med. Chem., 1995, 38, 2491.
- R. Weaver, I. H. Gilbert, Tetrahedron, 1997, 53,
5537.
- K. Haraguchi, Y. I. Tanaka, K. Yamaguchi, T. Miyasaka, J. Org. Chem.,
1996, 61, 851.
- U. Chiacchio, G. Gumina, A. Rescifina, R. Romeo, N. Uccella, A. Piperno,
G. Romeo, Tetrahedron, 1996, 52, 8889; H. J. Gi, Y.
Xiang, R. F. Schinazi, K. Zhao, J. Org. Chem., 1997, 62,
86.
- F. Casuscelli, U. Chiacchio, R. Ficarra, A. Rescifina, G. Romeo, Gazz. Chim. It., 1997, 267; U. Chiacchio, A. Piperno, A. Rescifina, G. Romeo, N. Uccella, Tetrahedron, 1998, 54, 5695.