The interpretation of these results is hampered by the well known problems of the AM1 method in the description of carbonyl - nucleophile reactions, especially the considerably overestimated stability of tetrahedral intermediates. Consequently, activation energies for processes leading to the decomposition of such tetrahedral intermediates are grossly overestimated. Furthermore, some of the tetrahedral intermediates obtained by the calculations are more stable than the reaction product 2 (see Table 3).
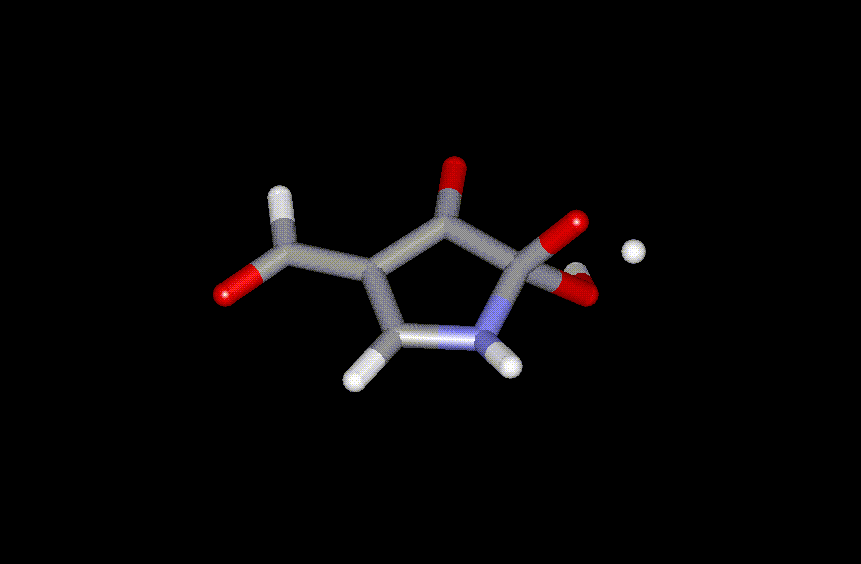
As an example, the transition state for elimination of H2O from the C2 - hydrate of 4-formyl-pyrrole-2,3-dione to 2 + H2O (the rate determining step ) is shown in Figure 4.
This mechanism is most likely on the basis of experimental observations, the calculations, however, are in favour of a bimolecular mechanism involving TWO molecules of the furandione. Whether the high activation energies found for the nucleophile - catalysed pathway are an artifact of the semi-empirical method or an intrinsic property of the molecules is still under investigation.