Cornforth - type Rearrangement
According to semiempirical calculations3
the Cornforth rearrangement4 of
e.g. 4-acyl- oxazoles occurs via ring opening and recyclisation of
a carbonyl-stabilised zwitterionic intermediate. For the present system
this mechanism implies formation of an a-oxo
acylkation.
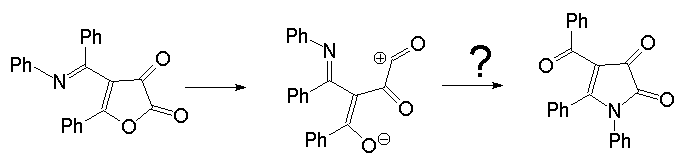
Such an intermediate is expected to be unstable; in fact, the calculation
for the ring opening of the furandion lacton ring lead to a transition
state for elimination of CO to give a-oxo ketenes
rather than cyclisation to the pyrroledione 2. The activation energy
for this elimination is ~ 30 kcal mol-1 . Thermal elimination
of CO from furan-2,3-diones is a useful method for the production
of a-oxo ketenes. 5
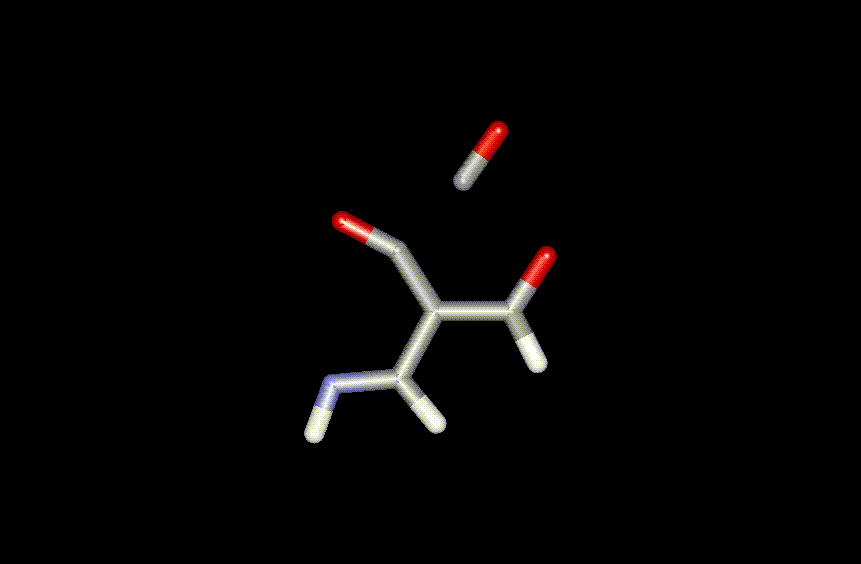
Figure 1. Transition state for lacton ring opening (leading to elimination
of CO rather than rearrangement).
You have to install the ChemScape
CHIME plugin with Netscape 3.0 (or higher) in order to see the embedded
images and to manipulate the displayed molecules "live" on your screen
Alternatively you can download freeware programs such as RASMOL
or WEBLAB VIEWER that can read
the *.pdb file.
Rearrangement 1 -> 2 via a Cornforth
- type mechanism appears thus highly unlikely.

