Mechanism of Halogen Dance Reactions

After initial metalation of 1 by LDA (Step {1}) via halogen-metal exchange reactions a series of rearrangement steps occur resulting in the formation of rearranged target I3. Two equations are mainly responsible for a complete turnover: {3} regenerates starting compounds 1 thus promoting continuous transbromination in {2}, whereas {4} - in an auto-catalytic sense - permanently forms the transbrominating dibromothienyl catalyst I4 under concomitant production of the target HD product I3. This auto-catalytic compound I4 - firstly emerging in {2}, is prominently responsible for a successful migration via its role in {3} and{4}. All compounds considered within this scheme are symmetric and - as having two reactive sites for HD available - we therefore aimed at double halogen migrations: this fact has been neglected in the above scheme for graphical simplification.
The driving force for all these HD reactions is the formation of the finally most stable Li-intermediate, with the Li atom residing at the most acidic C-H position. We have undertaken first preliminary ab initio calculations with the best available and most appropriate basis set for these purposes (all results have been checked by frequency analysis after DFT calculation).
Ab initio calculations in the HD area

ab initio Calculations with GAUSSIAN94
basis set: g 6-311+g(2d,p), method: b3lyp
Hardware: SGI PowerChallenge RISC computer
Computing CPU time per molecule: up to 26 hrs
Experimental Realisation
Preliminary studies on a model-compound comprising an acetylene group were undertaken, to ensure its neglecting effect to potential ortho-litiation (which indeed would be a strongly limiting factor to complete halogen migrations):

In a rough non optimized experiment only HD and HD-retained product accompanied by some unconverted material, but no substitution adjacent to the acetylene group was observed.
Halogen dance reaction on 1a:

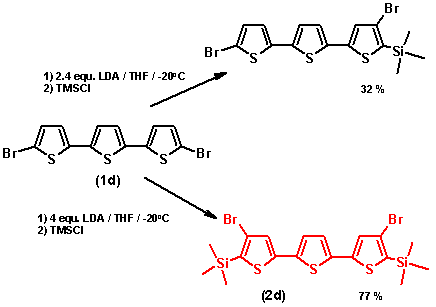
Halogen dance reaction on 1d:
The usually applied amount of 2.5 equ. LDA yielded only mono-rearranged product and left starting material. Increase of both
the amounts of LDA and of the solvent led to the desired product in good yields. This can be explained with the low solubility
of the starting product (1d) in THF and its low rate for lithiation: