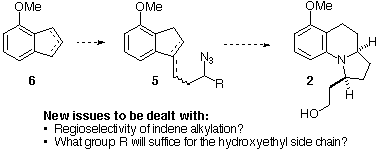
Scheme 4
Scheme 5. Attempt at Regioselective Alkylation of Methoxyindene 20.
Pure 23, the "wrong" regioisomer, was subjected to the Schmidt reaction (Scheme 6), producing 26 in moderate yield as a single regioisomer (i.e., no benzylic amine was observed). A sample of pure 22 could not be obtained by chromoatography, so a mixture of 22 and 23 was subjected to the Schmidt reaction. Compound 26 (derived from 23) was the only product; no 28 was detected, perhaps indicating that 22 does not undergo the Schmidt reaction. An alternate synthesis of a structure related to 22, the "correct" regiosomer, was developed to verify this conclusion.
Scheme 6. Schmidt Reactions of Regioisomeric Azido-Alkenes 22 and 23.
D. Regiocontrolled Routes to the Cyclization Precusors: Avoid
Indene Alkylations
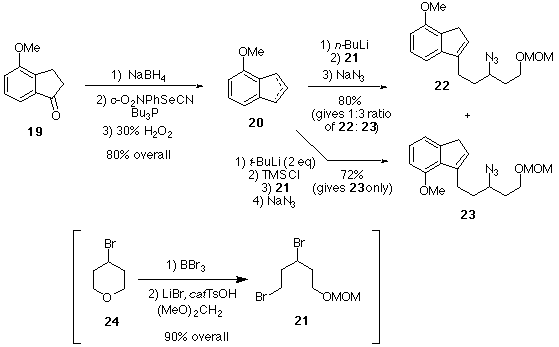
The related azido-alkene 32 was synthesized as shown in Scheme
7. Addition of dibromolithiomethane[Ref.25]
to the indanone 19 followed by treatment of the resulting adduct with zinc
dust[Ref.26] gave the vinyl bromide 29. Metal
halogen exchange on 29 followed by Lewis acid mediated opening[Ref.27]
of the epoxide 30[Ref.28] provided the alcohol
31 in good yield. Replacement of the alcohol with azide was accomplished with
a modified Mitsunobu reaction.[Ref.29] When the azido-alkene
32 was subjected to our usual Schmidt reaction/iminium ion reduction sequence,
no identifiable products were obtained, consistent with the observations in Scheme
6. Considering the data in Schemes 5 and 6 and the model
studies already described (i.e., no methoxy on the ring), we conclude that the
electron density in aromatic ring is an important influence in the success/regioselectivty
of the Schmidt migration. Regarding rate, we would expect the most electron rich
aromatic ring to migrate fastest, i.e. o-methoxyaryl (23/25, Scheme
6) > no methoxy (8/9, Scheme 2;
16, Scheme 3) > m-methoxyaryl
(22/27, Scheme 6 and 32/33, Scheme
7), which might explain why there is no cyclization for 22 and 32.
Regarding regioselectivity, the more electron rich aromatic ring will be better able
to compete effectively with alkyl migration. Thus we expect the ratio of aryl:alkyl
migration to decrease in the order given above. Indeed, the o-methoxyaryl
case is completely aryl selective, while the non-methoxy cases gave mostly aryl migration
(although the ratio was not high). The lack of success in the m-methoxyaryl
case does not allow us to discuss this data point.
Scheme 7. Schmidt Reaction of Azido-Alkene 32.
We next proposed that a side-chain lacking an oxygen atom might lead to a successful Schmidt reaction in the desired methoxyaryl case, since the azide would be slightly more nucleophilic. Thus, we chose to examine an allyl group, since this group might be transformed later into the desired 2-(hydroxy)ethyl group of gephyrotoxin. The synthesis and cyclization of such a compound, 36, is shown in Scheme 8. A cerium-mediated[Ref.30] addition of allylmagnesium chloride to methoxyindanone 19 gave an alcohol, which was to furnish a diene (not shown). Selective hydroboration of the terminal alkene with 9-BBN[Ref.31] followed by oxidation, provided the alcohol 34 in excellent overall yield. A one-pot Swern oxidantion/organometallic addition using Ireland's method[Ref.32] gave 35, which was transformed into the desired azide 36 with standard chemistry. Schmidt reaction of 36 followed by hydride reduction of the resultant iminium ion proceeded in excellent yield (86%) to produce a 1:1 mixture of regioisomeric products, 37 (desired) and 38 (undesired). Hence, the m-methoxyaryl group can migrate in good yield if a more nucleophilic azide is used (compare with Scheme 6 and Scheme 7), although the regioselectivity is poor, as predicted (vide supra). Nonetheless, oxidative cleavage of the alkene of 37 and reduction of the resultant aldehyde would complete a formal synthesis of gephyrotoxin by intercepting Ito's compound 2 (Scheme 1). However, all attempts at oxidative transformations on 37 were unsuccessful, leading to decomposition, perhaps due to the highly electron rich aromatic ring.
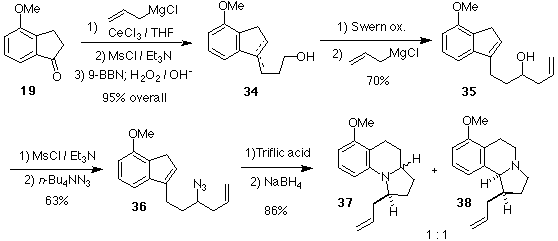
Scheme 8. Synthesis and Schmidt Reaction of Azido-Alkene 36.
Our search for the proper 2-hydroxyethyl equivalent had thus led us to uncover several important aspects of the Schmidt reaction required for the synthesis of gephyrotoxin, but each attempt had a flaw. However, we had now learned enough to narrow our search to a 2-haloethyl group, which would survive the acidic conditions of the Schmidt reaction, but would be easily transformed into a 2-hydroxyethyl group without relying on an oxidative reaction. A successful formal synthesis of gephyrotoxin is outlined in the next section.
1. Introduction
2. Model Studies: The Basic Schmidt Reaction,
wherein we study the regioselectivity of the rearrangement and the stereoselectivity
of the iminium ion reduction.
3. Initial Efforts to Synthesize Gephyrotoxin (THIS PAGE), wherein we struggle with the regioselectivity of the indene alkylation and the choice of a synthetic equivalent for the 2-hydroxyethyl side chain.
4. Formal Synthesis of Gephyrotoxin, wherein
we finally get it right.
5. References