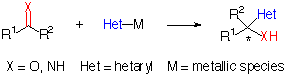

Nucleophilic addition of metalated heterocycles to C=X p bonds is a simple and common synthetic operation, and development of a chiral version leading to optically active compounds is obviously desiderable (Scheme 1) because of the broad range of biological activity possessed by these heterocyclic compounds[1] and their versatility as key synthetic intermediates[2]. A variety of heterocyclic systems including oxazolines[3], benzotriazole[4], furan[5] and thiazole[6] can be used as synthetic equivalents of several functionalities such as formyl and carboxyl groups.
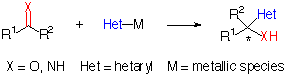
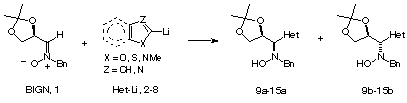
| Het- | additive | syn : anti | yield (%) | major adduct | [a]D (c, CHCl3) | mp (°C) |
| none
Et2AlCl |
92 : 8
3 : 97 |
82
84 |
9a
9b |
-7.8 (0.74)
-9.0 (0.39) |
oil
157-159 |
|
| none
Et2AlCl |
94 : 6
5 : 95 |
98
94 |
10a
10b |
-28.9 (0.79)
-13.2 (1.00) |
oil
119-120 |
|
| |
none
Et2AlCl |
88 : 12
21 : 79 |
81
76 |
11a
11b |
+17.2 (1.50)
-14.2 (0.88) |
103-105
118-120 |
| |
none
Et2AlCl |
>95 : 5
22 : 78 |
90
86 |
12a
12b |
-24.9 (0.41)
-14.6 (0.32) |
117-119
126-128 |
| |
none
Et2AlCl |
94 : 6
25 : 75 |
83
86 |
13a
13b |
-25.7 (0.36)
-31.2 (0.40) |
153-155
188-190 |
| |
none
Et2AlCl |
>95 : 5
>5 : 95 |
91
83 |
14a
14b |
-30.4 (0.26)
-22.3 (0.41) |
129-131
97-99 |
| |
none
Et2AlCl |
>95 : 5
30 : 70 |
88
84 |
15a
15b |
-35.2 (0.30)
-22.3 (0.60) |
102-104
127-129 |
 Experimental part
Experimental part 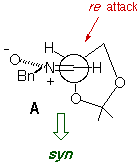
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Formula | C16H20N2O3S | C17H23N3O3 | C17H21NO3S | C20H22N2O3S | C21H23NO4 |
| M |
|
|
|
|
353.40 |
| space group |
|
|
|
|
|
| a (Å) |
|
|
|
|
|
| b (Å) |
|
|
|
|
|
| c (Å) |
|
|
|
|
|
| V (Å3) |
|
|
|
|
|
| Z |
|
|
|
|
|
| Dx (Mg m-3) |
|
|
|
|
|
|
|
|
|
|
|
|
| q range (°) |
|
|
|
|
|
| F (000) |
|
|
|
|
|
| m |
|
|
|
|
|
| No. of measured reflections |
|
|
|
|
|
| No. of unique reflections |
|
|
|
|
|
| R (int) |
|
|
|
|
|
| No. of reflections used (N) |
|
|
|
|
|
| No. of reflections with I > 2s(I) |
|
|
|
|
|
| No. of refined parameters (P) |
|
|
|
|
|
| Extinction parameter (SHELXL), q |
|
|
|
|
|
| wR2 = [Sw(DF2)2/Sw(Fo2)2]1/2 |
|
|
|
|
|
| wR2 for all data |
|
|
|
|
|
| S2 = [Sw(DF2)2/(N-P)]1/2 |
|
|
|
|
|
| R1 = S*DF*/S*Fo* for I > 2s(I) |
|
|
|
|
|
| R1 for all data |
|
|
|
|
|
| g (w=1/[s2(Fo2)+(g(Fo2+2Fc2)/3)2] |
|
|
|
|
|
| standard decay (%) |
|
|
|
|
|
Oearlier studies dealing with the addition of 2-lithiothiazole
and 2-lithiofuran to nitrones has been extended to other lithiated heterocycles.
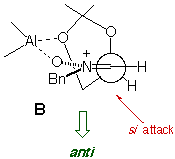
The absence/presence of Et2AlCl induces a dramatic change in
the stereochemistry of the reaction, and chiral syn and anti
heterocycle-containing hydroxylamines can be prepared in a stereocontrolled
way.
Application of this technology to the synthesis
of various heterocyclic compounds now becomes of interest, and further
synthetic applications of the obtained products are under investigation
in our laboratory.
[2] Shipman, M. Contemp. Org. Synth. 1994, 2, 1-17.
[3] Langlois, Y. Current Org. Chem. 1998, 2, 1-18
[4] Katritzky, A.R.; Lan, X.; Fan, W.-Q. Synthesis, 1994, 445-456 and references cited therein.
[5] For some selected recent examples see: (a) Dondoni, A.; Franco, S.; Junquera, F.; Merino, P.; Merchan, F.L.; Tejero, T. J. Org. Chem. 1997, 62, 5497-5507. (b) Kobayashi, Y.; Nakano, M.; Okui, H. Tetrahedron Lett. 1997, 38, 8883-8886. (c) Martin, S.F.; Barr, K.J. J. Am. Chem. Soc. 1996, 118, 3299-3300. (d) Piancatelli, G.; D'Auria, M.; D'Onofrio, F. Synthesis, 1994, 867-889. (e) Marshall, J.A.; Luke, G.P. J. Org. Chem. 1993, 58, 6229-6234.
[6] Dondoni, A.; Merino, P. Comprehensive Heterocyclic Chemistry. 2nd Edition. Vol. 3. Cap. 6. pp. 373-474. Katritzky, A.R.; Rees, C.W.; Scriven, E.F.V. (Eds.) Elsevier. 1996
[7] See for instance: (a) Tsutsumi, S.; Okonogri, T.; Shibahara, S.; Ouchi, S.; Hatsushiba, E.; Patchett, A.A.; Christensen, B.G. J. Med. Chem. 1994, 37, 3492-3502. (b) Yokoyama, M.; Toyoshima, H.; Shimizu, M.; Togo, H. J. Chem. Soc. Perkin Trans. 1, 1997, 29-33. (c) Hodges, J.C.; Patt, W.C.; Connolly, C.J. J. Org. Chem. 1991, 56, 449-452. (d) Reetz, M.T.; Holdgrun, X. Heterocycles, 1989, 28, 707-710.
[8] (a) Casiraghi, G.; Zanardi, F.; Rassu, G.; Spanu, P. Chem. Rev. 1995, 95, 1677-1716. (b) Dondoni, A.; Fantin, G.; Fogagnolo, M.; Merino, P. unpublished results.
[9] (a) Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1992, 33, 4221-4224. (b) Dondoni, A.; Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1993, 34, 5475-5478.
[10] (a) Dondoni, A.; Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1993, 34, 5479-5482. (b) Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Synthesis, 1994, 1450-1456.
[11] (a) Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T.; Bertolasi, V. Chem. Eur. J. 1995, 1, 505-520. (b) Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Scherrmann, M.-C.; Tejero, T. J. Org. Chem. 1997, 62, 5484-5496.
[12] (a) Lanaspa, A.; Merchan, F.L.; Merino, P.; Tejero, T.; Dondoni, A. Electronic Conference on Trends in Organic Chemistry (ECTOC-1). Rzepa, H.S.; Goodman, J.C. (Eds.). CD-ROM. Royal Societry of Chemistry Publications. 1995. ISBN 0-85404-899-5.(b) Merino, P.; Lanaspa, A.; Merchan, F.L.; Tejero, T. Electronic Conference on Trends in Organometallic Chemistry (ECTOC-3). Rzepa, H.S.; Leach, C. (Eds.). CD-ROM. Royal Societry of Chemistry Publications. 1997. ISBN 0-85404-889-8.
[13] (a) Dondoni, A.; Franco, S.; Merchan, F.L.; Tejero, T. Synlett 1993, 78-80. (b) Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1994, 35, 9439-9442. (c) Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Chem. Commun. 1995, 2127-2128.
[14] (a) Merino, P.; Anoro,
S.; Castillo, E.; Merchan, F.L.; Tejero, T. Tetrahedron: Asymmetry
1996, 7, 1887-1890. (b) Merino, P.; Castillo, E.; Merchan,
F.L.; Tejero, T. Tetrahedron: Asymmetry 1997, 8, 1725-1729.
(c) Merino, P.; Franco, S.; Merchan, F.L.; Tejero, T. Trends Org. Chem.
1998 (in press).
[15] (a) Merino, P.; Merchan,
F.L.; Tejero, T. Electronic
Conference on Heterocyclic Chemistry (ECHET-96). Rzepa, H.S.; Syder,
J.P.; Leach, C. (Eds.). CD-ROM. Royal Societry of Chemistry Publications.
1997. ISBN 0-85404-894-4. (b) Merino, P.; Lanaspa, A.; Merchan,
F.L.; Tejero, T. Tetrahedron: Asymmetry 1997, 8, 2381-2401.
(c) Merino, P.; Lanaspa, A.; Merchan, F.L.; Tejero, T. Tetrahedron:
Asymmetry 1998, 9, 629-646.
[16] Merino, P.; Franco, S.;
Merchan, F.L.; Tejero, T. Tetrahedron: Asymmetry 1997, 8,
3489-3496.
[17] Brandsma, L.; Verkruijsse,
H. Preparative Polar Organometallic Chemistry Springer-Verlag, Berlin,
1987.
[18] Tejero, T.; Franco, S.;
Junquera, F.; Lanaspa, A.; Merchan, F.L.; Merino, P.; Rojo, I. Tetrahedron:
Asymmetry 1996, 7, 1529-1534.
[19] Sheldrick, G.M. SHELXS-86
Program for Crystal Structure Solution. University of Gottingen, Germany,
1986.
[20] Sheldrick, G.M. SHELXL-93
Program for Crystal Structure Refinement. University of Gottingen, Germany,
1993.