C02 Chiral Amplification by Formation of meso-2:1 Complex (Mechanism 01)
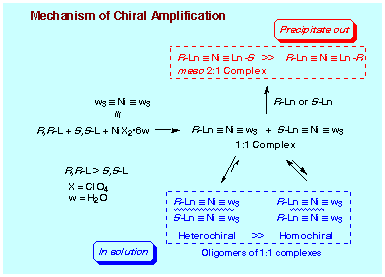
meso 2:1-Complex Formation (Mechanism 01):
Treatment of Ni(ClO4)2┤6H2O with the pure enantiomer
of DBFOX/Ph produces the active 1:1 complex, which is soluble in dichloromethane
at least at an early stage of complex formation. When the DBFOX/Phligand
of a low enantiomeric purity is used for complex formation, the pale blue
solid is rapidly precipitated regardless of presence of dienophile 3-acryloyl-2-oxazolidinone.
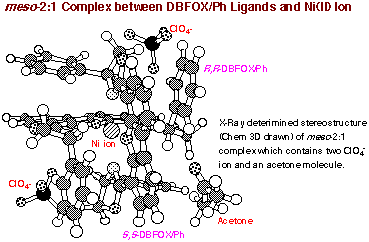
This product has a molecular formula of the monohydrate of (DBFOX)2┤Ni(ClO4)2
(by elemental analysis) consisting of a pair of heterochiral DBFOX/Ph ligands
(R,R- and S,S-DBFOX/Ph). A CD spectrum recorded in 1,2-dichloroethane containing
a small amount of DMSO shows no absorption. Accordingly, the structure can
be assigned to be an S4-symmetric meso structure. This unusual structure
of meso-2:1 complex was ultimately confirmed by X-ray single crystallography
analysis. The most straightforward access to meso-2:1 complex is the treatment
of 1:1 adduct R,R-DBFOX/Ph with the other enantiomer of free ligand S,S-DBFOX/Ph,
and this actually takes place quantitatively.

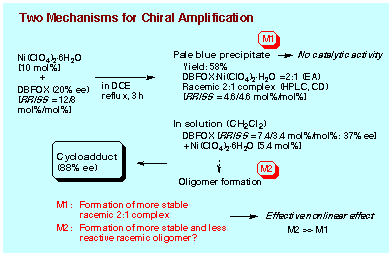
By irreversible formation of meso-2:1 complex, the major enantiomer of DBFOX/Ph can be enriched in the solution so that the enantioselectivity for endo-cycloadduct should exceed the enantiomeric purity of the ligand used. However, even when the complex formation is performed under reflux in 1,2-dichloroethane to effect the 2:1 complex formation (RR-DBFOX/Ph: 12 mol%, SS-DBFOX/Ph: 8 mol%, Ni(ClO4)2┤6H2O: 10 mol%, 3 h), the yield of meso-2:1 complex was only 58% yield. Enantiomeric purity of DBFOX/Ph ligand remaining in the solution is calculated to be 37% ee (RR-DBFOX/Ph: 7.4 mol%, SS-DBFOX/Ph: 3.4 mol%, Ni(ClO4)2┤6H2O: 5.4 mol%), while the enantioselectivity actually observed for endo-cycloadduct was 88% ee. Apparently a chirality enrichment mechanism other than that through the precipitation of meso-2:1 complex exists in the solution. We have not so far observed that the 1:1 complex DBFOX/Ph┤Ni(ClO4)2┤3H2O can be dissociated into each component under the usual reaction conditions. It is improbable that the 1:1 complex as active catalyst undergoes disproportionation leading to the inert meso-2:1 complex. Accordingly, the formation of meso-2:1 complex has to proceed only through the reaction of 1:1 complex with free DBFOX/Ph ligand, and hence this transformation is limited to occur in an early stage of complex formation. When all the free ligand DBFOX/Ph is consumed, the production of meso-2:1 complex ceases.
Stability of meso-2:1 Complex:
The meso-2:1 complex is sparingly soluble in most organic
solvents, but it has limited solubility in DMSO and DMF which still show
limited solubilities. No decomposition occurs even on heating in wet DMSO
or DMF. Accordingly, once meso-2:1 complex is formed in the step of complex
formation, no dissociation into components, DBFOX/Ph and DBFOX┤Ni(ClO4)2,
takes place, formation of meso-2:1
complex being irreversible. Meso-2:1 complex is stable
even in the presence of excess 3-acryloyl-2-oxazolidinone used as chelating
reagent in the Diels-Alder reaction. The following experiments provide some
supporting evidence for the inactivity of meso-2:1 complex:
(1) When the isolated 2:1 complex (4 mol%) is
pretreated with a mixture of Ni(ClO4)2┤6H2O (16 mol%) and the pure enantiomer
of R,R-DBFOX/Ph (2 mol%) in dichloromethane, a high chiral induction is
recorded (97% ee for endo-cycloadduct) in the Diels-Alder reaction at -40
┴C. This selectivity is comparable to that observed in the reaction under
the catalysis of 2 mol% of enantiomeric pure R,R-DBFOX/Ph.
(2) The Diels-Alder reaction is not accelerated by a
mixture of the isolated meso-2:1 complex (4 mol%) and the pure enantiomer
of R,R-DBFOX/Ph (5 mol%).