C01 Chiral Amplification of the Diels-Alder Reactions Catalyzed by DBFOX/Ph Complexes
Expectation of Chiral Amplification:
R,R-DBFOX/Ph is a tridentate ligand that coordinates
strongly to nickel(II) perchlorate hexahydrate to form the characteristic
planar structure with the molecular formula of DBFOX/Ph´Ni(ClO4)2´3H2O.
The three water ligands located at meridional positions may be replaced
by another molecule of DBFOX/Ph ligand if steric hindrance is negligible.
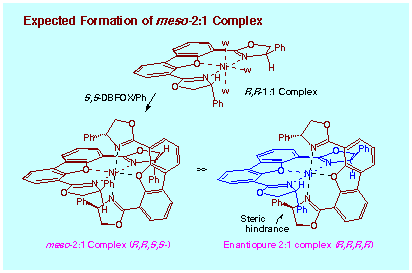
Based on molecular model inspection, the heterochiral enantiomer S,S-DBFOX/Ph
looks like a candidate to replace the water ligands to form the heterochiral
2:1 complex (DBFOX/Ph)2´Ni(ClO4)2 (R,R,S,S- or meso-2:1 complex). However,
the 2:1 complex containing a homochiral pair R,R,R,R-2:1 complex can be
never formed because of the severe steric hindrance between the 4-phenyl
substituents of oxazoline rings. When a catalyst complex is prepared from
DBFOX/Ph ligand of a low enantiomeric purity, formation of heterochiral
meso-2:1 complex is expected, and that the resulting complex meso-2:1 complex
with saturated coordination should be inert in catalytic activity. If this
happens, part of minor enantiomer of DBFOX/Ph ligand is consumed to enrich
enantiomeric purity of the remaining ligand, indicating a possibility of
effective chiral amplification. Accordingly, we have examined some experiments
of chiral amplification in the Diels-Alder reaction catalyzed by a complex
between DBFOX/Ph and Ni(ClO4)2´6H2O.

Effective Chiral Amplification:
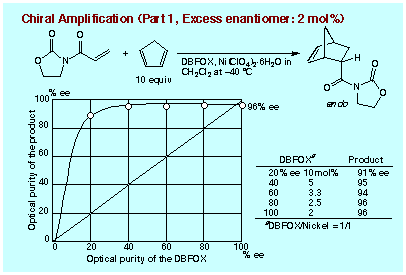
A typical procedure is as follows: a catalyst was prepared in situ
from Ni(ClO4)2´6H2O (20 mol%) and DBFOX/Ph of a low enantiomeric purity
(20% ee, R,R-DBFOX/Ph: 12 mol%; S,S-DBFOX/Ph: 8 mol%) under stirring in
dichlomethane at room temperature for 2 h. During this procedure, the nickel
salt becomes gradually dissolved in the solution, but at a late stage of
this procedure some pale blue solid starts to precipitate. Without removal
of this solid, the resulting suspension was employed in the Diels-Alder
reaction between cyclopentadiene (10 equiv) and 3-acryloyl-2-oxazolidinone
at -40 ĮC for 72 h. Cycloadduct was obtained in 95% yield (endo:exo = 97:3)
with an enantioselectivity as high as 96% ee for endo-isomer. Figure shows
the relationship between the enantiomeric purity of DBFOX/Ph ligand used
and the enantioselectivity observed for endo-cycloadduct, where the ratio
of DBFOX/Ph vs Ni(ClO4)2´6H2O is 1:1 mol/mol and a catalytic loading is
2 mol% for the excess enantiomer of DBFOX/Ph. With the DBFOX/Ph of 20% ee,
a 91% ee was recorded for endo-cycloadduct, and 95% ee was recorded from
40% ee ligand. One should recognize that the excellent levels of chiral
amplification have been attained, since the maximum enantioselectivity is
96% ee (endo-cycloadduct) which can be attained in the reaction using the
pure enantiomer of DBFOX/Ph ligand (2 mol%) at -40 ĮC. This means that the
reaction has been catalyzed by the almost pure enantiomer of complex DBFOX/Ph´Ni(ClO4)2´3H2O
which remained in the solution by an absolutely effective chirality enrichment
process.