B05 Transition Sturucture of DBFOX/Ph Catalyzed Diels-Alder Reactions
Simplicity of Transition Strucure:
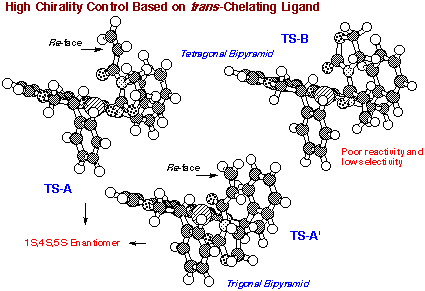
Based on molecular model inspection of the possible
transition state structures of R,R-DBFOX/Ph catalyzed asymmetric Diels-Alder
reaction, the Re-face of the unsaturated bond of dienophile should be the
enantioface participating in the catalyzed reaction. This prediction was
verified by experiment: the absolute configuration and enantiomeric purity
of endo-cycloadduct were determined by optical rotation and the chiral HPLC
analysis (Daicel Chiracel OD with hexane:2-PrOH = 99:1 v/v), respectively.
The assigned structure of endo-cycloadduct is (1S,2S,4S)-3'-(bicyclo[2.2.1]hept-5-en-2-yl)-2'-oxazolidinone.

Square Bipyramidal Complex:
We propose that the reacting catalyst - substrate (dienophile)
complex between R,R-DBFOX/Ph´3H2O and 3-acryloyl-2-oxazolidinone is a square
bipyramidal structure containing an octahedral nickel ion. Although there
is an evidence for the existence of two coordination structures, A and B
in solution, the sterically more hindered or p-stacking B should be less
reactive than A. Therefore, we suggest that complex B is responsible for
the highly selective reaction on the re-face with respect to the "alpha"-carbon
of dienophile. This analysis of the transition state structure is consistent
with the observed absolute configuration of the endo-cycloadduct. Coordination
structures such as A and B are the only possible substrate complexes. This
structural simplicity is an important advantage of DBFOX/Ph complexes over
the traditional cis-chelating bisoxazoline ligands, where a number of possible
substrate complexes make it difficult to analyze the transition state structure.