B04 Competitive Coordination between DBFOX/Ph and Substrates
Competitive Coordination:
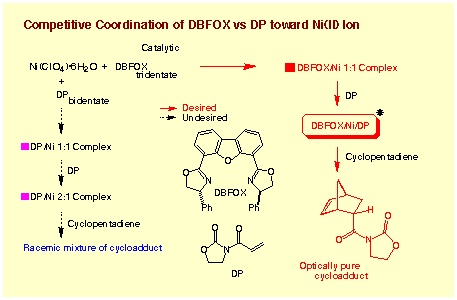
In the substrate complexes, DBFOX/Ph´Ni(ClO4)2´3H2O + 3-acryloyl-2-oxazolidinone,
three kinds of ligands are coordinating onto the nickel ion: chiral DBFOX/Ph
ligand R,R-1. The ligand (DBFOX/Ph) and the substrate (3-acryloyl-2-oxazolidinone)
should compete each other, and this
competition poses a general and serious problem in
the reactions catalyzed by complexes of neutral chiral ligands. If the substrate
is a better ligand than the ligand, then effective catalysis is not expected.
The results suggest that DBFOX/Ph is a much better ligand than the substrate
in this case. Formation of the tight
complex is one of the most attractive features of tridentate DBFOX ligands. The nitrogen - nitrogen distance of 4.1 A ensures the high stability
of nickel complex. As mentioned above, the solubility of Ni(ClO4)2´6H2O
in dichloromethane is very low in the absence of DBFOX/Ph, and the dienophile
itself does not solubilize the nickel ion at all. No rate acceleration is
observed in the Ni(ClO4)2´6H2O catalyzed Diels-Alder reaction in the absence
of DBFOX/Ph. Accordingly, excess amounts of Ni(ClO4)2´6H2O can be employed
in the reaction without loss of enantioselectivity; the amount of nickel
ion necessary for the 1:1 complexation is extracted by DBFOX/Ph ligand into
solution. This will be discussed in detail in the section on chiral amplification.
Such improved solubility is again no doubt due to the exceptionally high
stability of DBFOX/Ph´Ni(II) complex. On the other hand, nickel complexes
of the known trans-chelating pybox/Ph ligand, both aqua and anhydrous complexes,
show much lower selectivities.
