Rates of the Meisenheimer rearrangement for the different isomers
An interesting observation was noted in the rates of the N-oxide rearrangements. It was found that the rate of rearrangement of 6 was much higher than the rate of rearrangement of 5 on refluxing in butyronitrile.
The reason for the difference in rates is unclear. Interaction of the double bond and the negatively charged oxygen, might be expected to influence ground state stability but factors affecting the biradicaloid transition state in each case remain to be clarified.
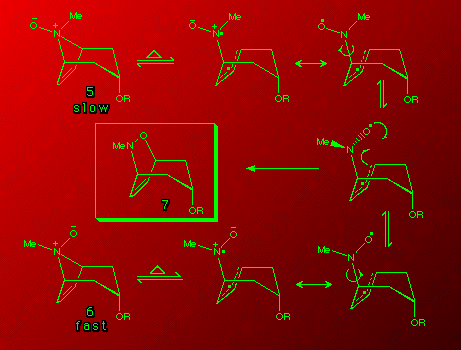
Preliminary ab initio calculations6 do not show a considerable difference in ground state stability. What they do show is that 6 is more stable than 5 by 6.65 kcal mol-1 (1 cal = 4.184 J). This would imply that ground state stability does not play a role in the rate of rearrangement. Work has begun on calculations of the transition state energies.
Further comment or discussion on the difference in rates would be most welcome.
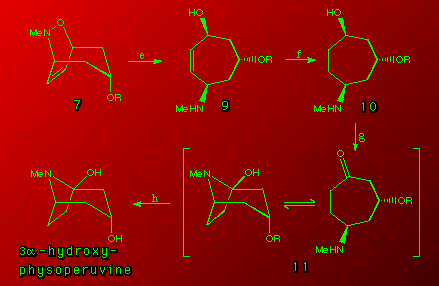
Final synthetic steps
The final steps were fairly straightforward. Ring cleavage of 7 with Zn/acetic acid (e) gave 9 in 92% yield. Hydrogenation of 9 (H2/Pd-C) (f) gave a quantitative yield of 10. Oxidation of 10 with pyridinium chlorochromate yielded 11 (g) which on deprotection (3% aqueous HCl) (h) gave the final compound 3-a-hydroxyphysoperuvine in a 67% yield from 10. A single crystal X-ray analysis has established structure 11 as the bicyclic isomer in the solid state ( A. H. White, unpublished work).
Conclusion
We have demonstrated a novel synthetic route to a new bridgehead hydroxylated tropane derivative using Meisenheimer rearrangement technology. This should be extendable to other appropriately substituted nitrogen bridged systems to allow access to a large range of new bridgehead hydroxylated compounds. Also we have demonstrated a new high yielding two step route to trop-6-en-3-a-ol (3)7. This compound could be used in the synthesis of a range of other tropane alkaloids and derivatives as well as in the study of biosynthetic pathways via labelling of the double bond.
- Asano, N.; Kato, A.; Oseki, K.; Kizu, H.; Matsui, K. Eur. J. Biochem., 1995, 229, 369.
- Ray, A. B.; Oshima, Y.; Hikino, H.; Kabuto, C. Heterocycles 1982, 19, 1233.
- Moore, J. M.; Hays, P. A.; Cooper, D. A.; Casale, J. F.; Lydon, J. Phytochemistry 1994, 36, 357.
- Kupchan, S. M.; Maruyama, M. J. Org. Chem. 1971, 36, 1187.
- Bremner, J. B.; Smith, R. J.; Tarrant, G. J. Tetrahedron Lett. 1996, 37, 97.
- Geometry Optimization, RHF/STO-3G, SPARTAN Ab Initio Program: SGI/R4K, Release 4.0.2b by Wavefunction, Inc.
- Hayakawa, Y.; Baba, Y.; Makino, S.; Noyori, R.; . J. Am. Chem. Soc.. 1978, 100, 1786.


