An outlook on synthetic applications: Ring opening reactions on HD-Bithiophenes
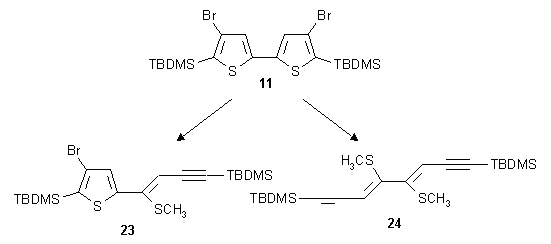
The double-migration products were successfully converted to new ene-ynes via
metal/halogen exchange with BuLi and consecutive ring-opening reaction, by allowing the
Li-intermediates thus obtained to warm-up to room temperature. The metalated intermediates were
quenched with e.g. CH3I.
Under appropriate conditions these ring-opening reactions proceed without any by-products.
In ongoing projects these ene-ynes should be investigated as substrates for selective reduction of the
triple-bonds. Due to the ease of the introduction of a variety of substituents via HD-methodology
a straightforward approach to a series of new substituted dienes (applicable in electrocyclic
chemistry) should be given.

