Mechanistic considerations
HD-reactions are sometimes referred to base-catalysed
halogen-dance (BCHD) reactions [1,2],
should be considered as
aryl-bromide catalysed dance (ABCD) reactions, because the driving
force of such rearrangements is the generation of polybromoaromatic intermediates, which serve
as transbromination catalysts within a series of metal-exchange reactions, as depicted below
(5,5'-dibromo-2,2'-bithiophene 1 used as starting compound).
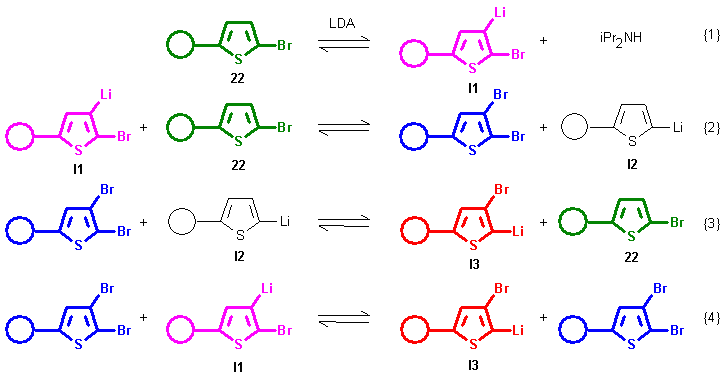
After initial metalation of 1 by LDA (step [1]) a series of
rearrangement steps occur (step [2], [3], [4]) which result in formation of rearranged I3.
(Although 5,5'-dibromo-2,2'-bithiophene 1 has two reactive sites, this fact is neglected
for simplification in the previous scheme).
From the proposed mechanism it can be easily deferred that formation of rearranged product
requires contact of starting compound 1 with the intermediate I1 which can
in practise be achieved by
- providing excess of starting material
- rapid addition of starting material
- elevated reaction temperature (approx. -20 °C)
- reduction of the polarity of solvent (which causes [1] to react more slowly)
- inverse addition of base
Usually, application of conditions 1 - 4 enables successful formation
of desired I3.
In contrast, opposite measures have to be taken to obtain non-rearranged products.

