Dihydro-oxazine derivative
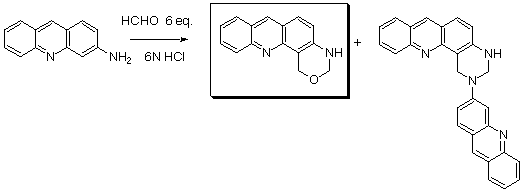
Reaction of 3-aminoacridine with an excess of formaldehyde in 6 M HCl yields a mixture of two compounds:
the dihydro-oxazine and the dihydro-quinazoline. The ratio between these compounds depends on many factors
(temperature, concentration in both reactants, stoechiometry). To achieve the selective synthesis of the
dihydro-oxazine, it is necessary to mono-protect the amino group with an ethoxycarbonyl group. Reaction with
formaldehyde gives a protected oxazine that is readily deprotected.
The dihydro-oxazine ring can be opened by DDQ oxidation to give the carbonyl derivative or by reduction into
the ortho-methylamino hydroxymethyl derivative.
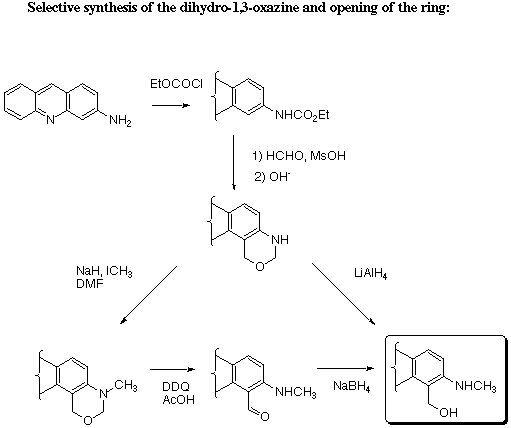
The 3-methylamino-4-hydroxymethyl acridine has been studied in a programme devoted to the synthesis and study
of new anticancer drugs.



