Formation of aza-kekulene or aza-helicene
We made a first attempt on the reaction between a diamino triaza-heptacycle and a proflavine in the
presence of formaldehyde in HCl. After a week of stirring , the solution was basified and the
resulting solid was analyzed by proton NMR (TFA-d) and mass spectrometry. The results indicated the presence of
the postulated hexa-aza-kekulene (M = 606) along with a diamino-hepta-aza-helicene (M = 815) resulting from
the condensation of two heptacyclic molecules.
The molecular models of both compounds are shown below. After minimization the aza-kekulene appeared
curved like a "flying saucer".
We are now working on the purification of the two compounds. Due to their very low solubility, this appears to be a real challenge.
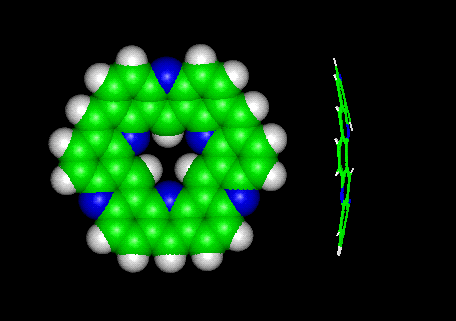
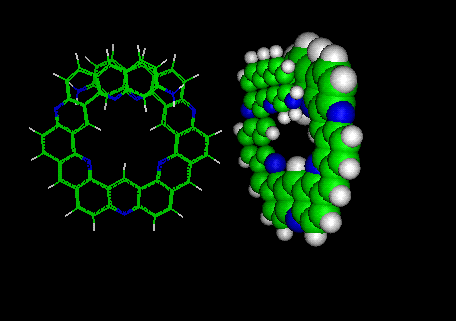
Molecular modelling was done with the INSIGHT/DISCOVER programs from Biosym-MSI Inc.




