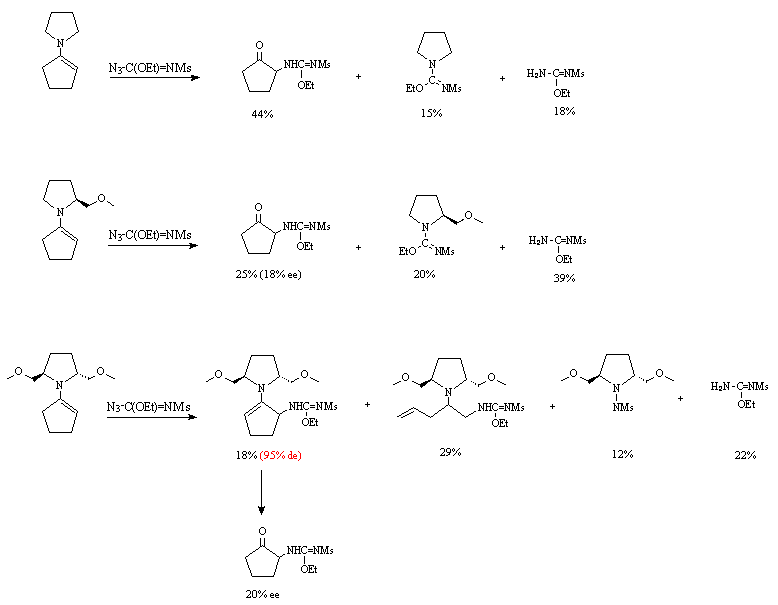

We should stress the novelty of the first two products derived from the last enamine and particularly the very high de of the first and the unprecedented ring opening product.
We believe the hydrolysis step might be in all cases responsible for the observed partial racemisation.
