Conclusions
The chosen azide showed as good reactivity towards sterically hindered cyclohexanone enamines, as that derived from trans-2,5-di(methoxymethyl)pyrrolidine, while ethyl azidoformate failed to react with this last enamine.
In contrast, the corresponding cyclopentanone enamines gave the expected N-substituted a-aminocyclopentanone.
A common intermediate seems to be a triazoline, which follows different pathways depending on the enamine structure.
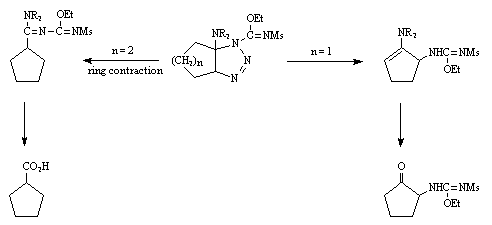
We believe the cycloaddition giving the triazoline to proceed with the same stereochemistry as analogous cycloadditions.7
Triazoline 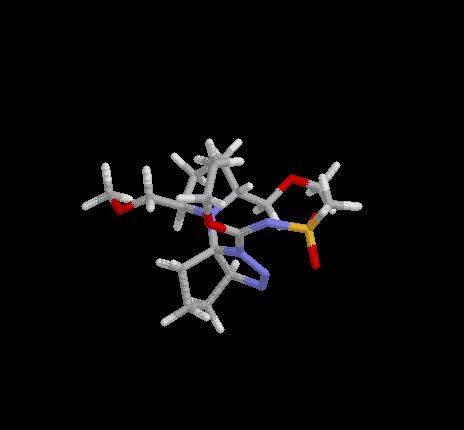 .
.



