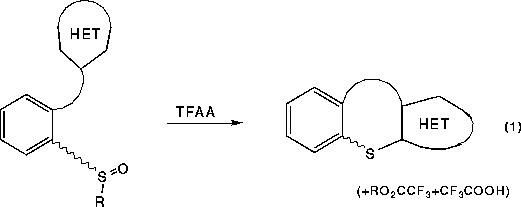

This general reaction, which takes place under traditional Pummerer rearrangement
reaction conditions (TFAA), produces sulfenylation products cleanly in good to
excellent yields when R is a simple alkyl group (R = CH3, C2H5). This process
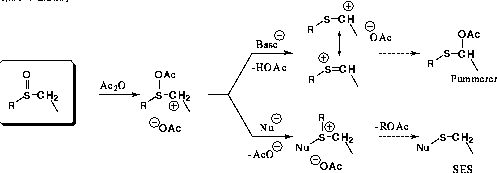
is thought to proceed via a mechanism similar to the Pummerer rearrangement. [2] except that nucleophilic attack occurs at sulfur before
the elimination reaction to a thionium ion for the Pummerer rearrangement pathway
takes place (eqn. 2). In all cases we have examined to date, the nucleophilic
source has been the pi-electron rich pyrrole ring, tethered in some fashion
to the sulfoxide moiety.
 Back to abstract
Back to abstract