

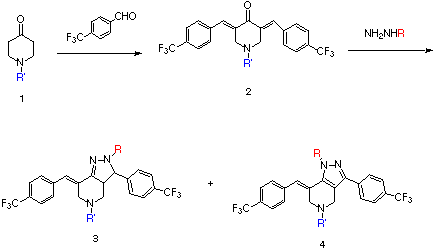
The piperidone (1) was reacted with 2 equiv. of 4-trifluoromethylbenzaldehyde to produce 2 in high yield. Treatment of 2 with mono-substituted hydrazines provided 3. When the hydrazine substituent was either a bulky aliphatic group or an aryl group, we often obtained some 4 as a byproduct. For example, when NH2NHCH(CH3)2 was reacted with 2 (R' = CH2CH2OCH3), 3 and 4 were obtained in an 85:15 ratio.
When NH2NHCH2CF3 was reacted with 2, however, the major product was 4. This posed a problem for the mixture synthesis (see Figure 1).