

Computational,
Theoretical
and Structural Chemistry
Department of Chemistry
Imperial College London
Room 134
Exhibition Road, London
SW7 2AZ
Phone:+44 (0)207 594 5871

The behaviour of fluids inside nano pores is of great interest in science and technology. The understanding of the structure, dynamics, and thermodynamics of these systems is of relevance to different disciplines. Nanotribology (lubrication, friction and wear), adhesion phenomena, pressure solution and crystallisation in pores are some of the wide range of situations in which confined fluids are important.
My research focuses on the application of the Molecular Dynamics (MD) computational technique for the study of fluids confined between solid surfaces. MD is a simulation technique based on the solution of Newton's equations of motion. It provides information for the calculation of both equilibrium and transport properties of the system under study. In my Ph.D, I have been employing MD for the study of different systems which have in common the fact that a fluid is confined by solid surfaces. A snapshot of one of the systems I study can be much more explanatory:
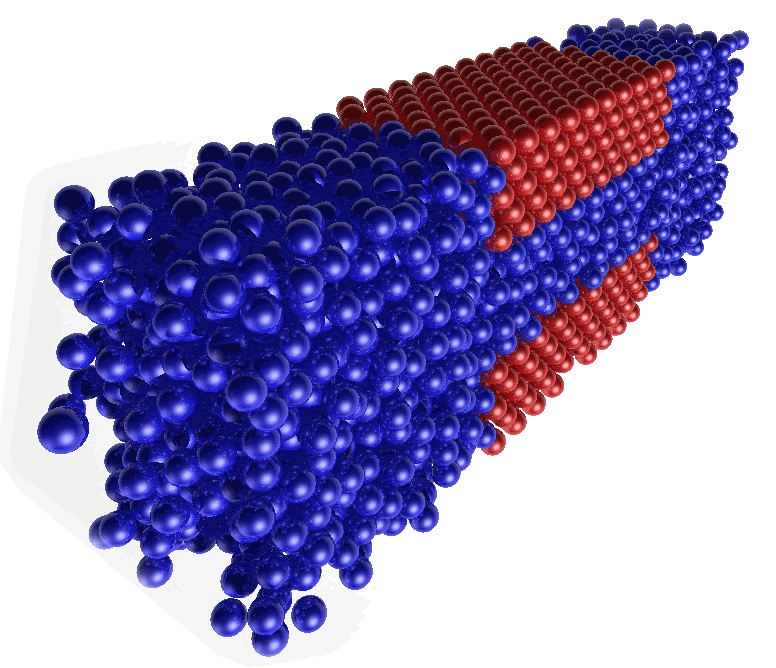
In the figure above, the red balls represent the atoms belonging to the solid surfaces, which confine the liquid between them (blue balls). On the other hand, the blue balls outside the confining region can be considered as a bulk liquid, which ensures equilibrium conditions along the system.
Properties inside the pore such as the normal pressure exerted by the confined fluid on the solid surfaces can be measured as a function of the surface-surface separation. Experimentally, this system is equivalent to the SFA (Surface Force Apparatus), so comparisons can in some cases be done between simulation and experimental results.
The study of the crystallisation of the liquid inside the confined region is one of the main aims of this research. I have study the influence of different factors such as the geometry of the pore and the structure of the confining surfaces. Both seem to be crucial in determining the behaviour of confined fluids and their crystallisation.
Since the start of my Ph.D, we have published two articles:
" Molecular Dynamics Simulations of crystallisation under confinement at triple point conditions", L.G. Cámara and F. Bresme, J. Chem. Phys., 119, 2792 (2003)." Liquids confined in wedge shaped pores: Nonuniform pressure induced by pore geometry", L.G. Cámara and F. Bresme, J. Chem. Phys., 120, 11355 (2004).
In the first paper, we observe the crystallisation of a simple fluid (Argon) confined between two parallel surfaces as a function of their separation and the temperature. We identify the force of crystallisation as the force exerted by the confined liquid on the solid surfaces when crystallisation occurs. The magnitude of this force turns out to be considerably large.
In the second paper, we employ a non parallel geometry and investigate its consequences. The conclusion is that the geometry determines many of the thermodynamic properties of the confined fluid.
In athird article, which is currently being written, we investigate the influence of the structure of the confining surfaces on the properties of the confined fluid. In this article we extend the work done in the two previous articles and try to give a clear picture of the different factors which determine the properties of confined fluids.
In the final year of my Ph.D, I will move into more complex systems, such as water confined by solid NaCl. In this case the NaCl will be allowed to dissolve so I will try to reproduce dissolution-precipitation processes in confining conditions.