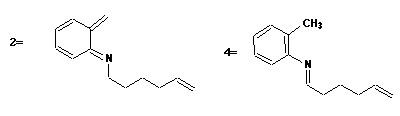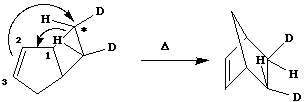The following are answers to a set of practice problems in Pericyclic Mechanisms.
They were prepared by Henry Rzepa for the
second year course at the Department of Chemistry, Imperial College.
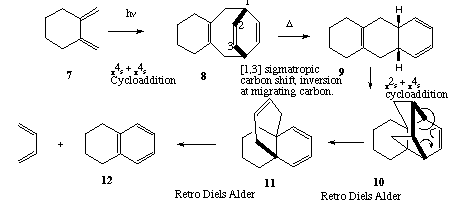
Qu. 1. Cycloelimination of CO2 from 1 gives the transient species 2 (4n+2 electrons, Huckel transition state,
thermally allowed). This is followed by either a [1,5] sigmatropic hydrogen shift (4n+2 electrons, Huckel t.s.,
thermal) to give 4, subsequently hydrolysed to 5, or by a p2s + p4s intramolecular cycloaddition to give the
other product 3. 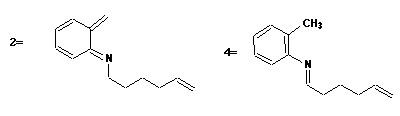
Back to Problems
Qu. 2. Formed by cycloelimination of CO2 from A, via a 4n+2 electron Huckel transition state (thermally allowed). B
then undergoes a 4n+2 electrocyclisation (Huckel t.s.) utilising either of the two single (alkene like) bonds of
the three membered ring to form C or D. These can then undergo a 4n cycloelimination of PhCN (thermal,
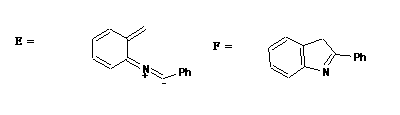
therefore must proceed via a Mobius transition state with one antrarafacial component) to give E.
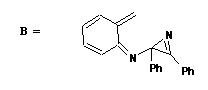
E can undergo an electrocyclisation (4n+2, Huckel, thermal) to give F, which can aromatise by TWO
consecutive [1,5] sigmatropic hydrogen shifts (4n+2, Huckel, thermal) to give the final indole product G.
Back to Problems
Qu. 3. a) This is an example of the "Domino Diels Alder Reaction", L. A. Paquette et al, J. Am. Chem. Soc.,
1978, 100, 5845.
b) J. Chem. Soc., Chem. Commun, 1988, 777.
Qu. 4. The preparation of "felicene", A. Gilbert and R. Walsh, J. Am. Chem. Soc., 1976, 98, 1606.
Back to Problems
Qu.5. a) The reaction is a [1,3] sigmatropic migration occuring thermally with 4n (n=1) electrons. Hence one
antarafacial component must be present, and inversion at the starred atom occurs, resulting in the observed
stereochemistry.
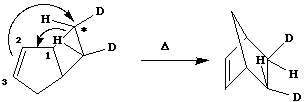
b) The absence of both the cyclopropyl and carbonyl groups implies cleavage of the 3-ring, most probably
via either [1,3] or [3,5] sigmatropic migration. The presence of only one non-vinylic proton (at d3.76) in the
nmr rules against the [1,3] migration and in favour of [3,5] thermal migration (4n electrons and hence one
antarafacial component). Models show that this can be easily accomodated on the 3-atom component if bond
a forms to the bottomface of the carbonyl p system and bond b breaks from the top face of the C(Ph)-C=O
system. Both bonds form/cleave from the top face of the 5-atom component as shown below. This results in
the cis ring junction stereochemistry.
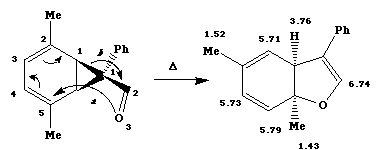
See P J Battye, D W Jones and H P Tucker, J. Chem. Soc., Chem. Commun.,1988, 495.
Back to Problems
Qu. 6. DMAD is a reagent that reacts readily with dienes. Two independent 4n+2 electron thermal
electrocyclic reactions convert ditropyl into two separate diene units, followed by 4n+2 thermal cycloaddition
of the DMAD to give [B]. Four different stereoisomers are possible, but the two unsymmetrical forms are
ruled out from the nmr evidence. Distinction between the two symmetrical forms could have been made on the
basis of the coupling constant (3Hz), which is known from other examples to be ca 4Hz for the isomer shown
and ca 9Hz for the alternative isomer. A double 4n+2 cycloelimination gives rise directly to [C] and [D]. [C] is
an isomer of benzene, to which it rearranges via probably homolysis of the cyclopropyl bond.
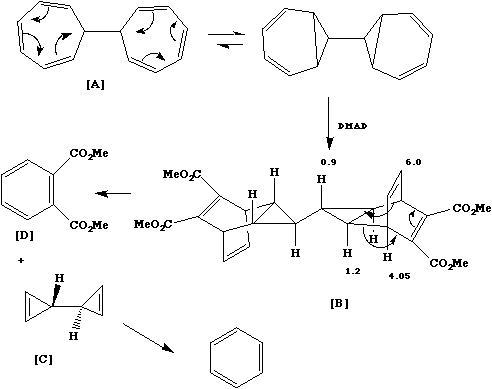
Back to Problems
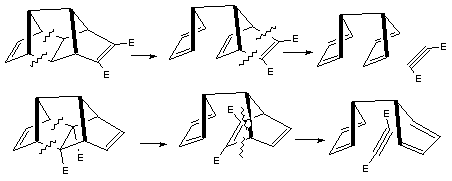
Qu 7. a) Comparison between reactant and product suggests they differ only in the position of one hydrogen (by 5
carbon atoms) and a C-C bond;
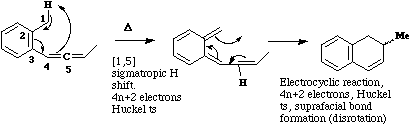
Back to Problems
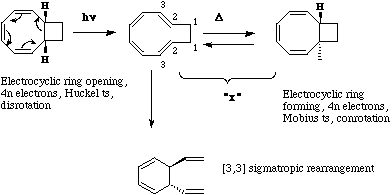
Qu. 8. The reversible nmr behaviour suggests an equilibrium between two species, involving the
interconversion between an alkene (d 6.3-4.8) and an alkane (d3.3-1.4). At high temperatures, the chemical
shift of two hydrogen is averaged to the mean position (d3.82). Hydrogenation 'traps' out the 'alkane', whilst
heating 'traps' out the alkene by further (Cope) reaction.
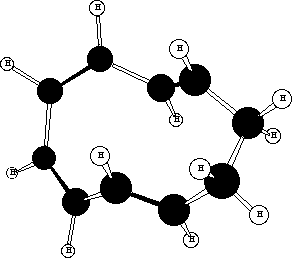
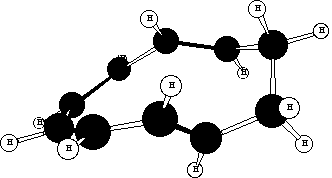
A number of cis/trans isomers for the 10-ring cycloalkene are possible, of which models suggest one in which
the new double bonds are formed trans is most likely (see below).The trans form can exist in two different
conformations, for both of which the s bond cleaving and the s bond forming are suprafacial on each C3
fragment of the [3,3] sigmatropic rearrangement. The conformation resulting in trans stereochemistry for 6
must be the more stable;
When this species cyclises to 7, this conformation sets the bond up to form from the bottom face of one end of
the octatetraene component, and the top face of the other end, to form the trans stereochemistry in the 4-ring.
The other possible conformation appears less likely to result in the required stereochemistry;
The conformation where the two double bonds are formed cis looks like this;
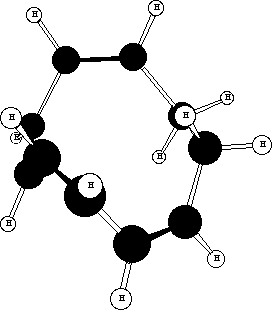
A quantitative estimate of the relative stability of these three forms can be obtained by "molecular mechanics"
modelling (ie using the MODEL program). Their energies are 114, 139 and 135 kJ mol-1 respectively, which
conforms that the stereochemistries of the products are controlled by the conformational stability of the
intermediate 10-ring.
Back to Problems
Qu. 9.
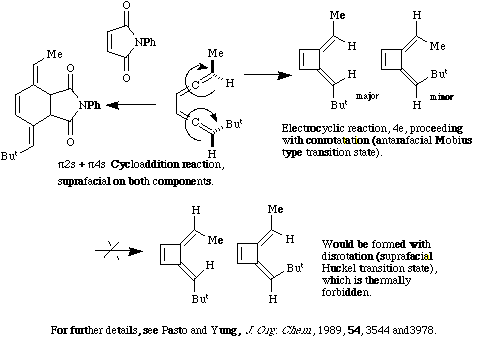
Back to Problems
Qu. 10.
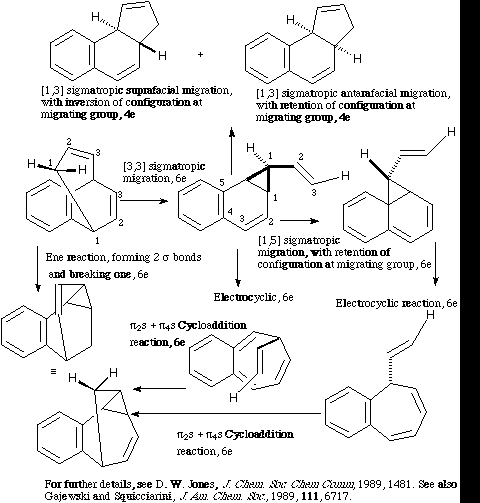
Back to Problems
Qu 11. a)
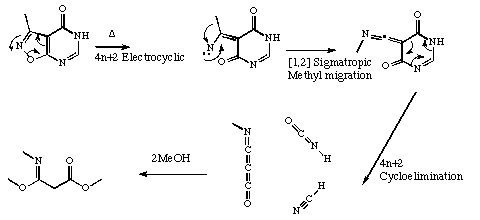
Y. Himeda, K. Hiratani, M. Hatanaka and I. Ueda, J. Chem. Soc., Chem. Commun, 1992, 1684.
b)

T. Mosandl, C. O. Kappe, R. Flammang and K. Wentrup, J. Chem. Soc., Chem. Commun, 1992, 1571.
Back to Problems
Qu 12,
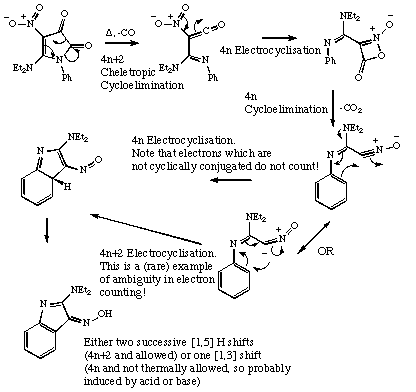
C. O. Kappe, G. Kollenz and K. Wentrup, J. Chem. Soc., Chem. Commun, 1992, 485.
Back to Problems
Qu 13.
Back to Problems
Qu 14. See K. Harano, M. Eto, K. Ono, K. Misaka and T. Hisano, J.
Chem. Soc., Perkin Trans 1, 1993, 299 for further details.
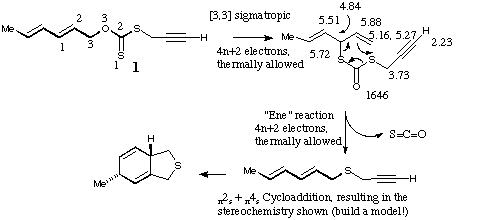
Back to Problems
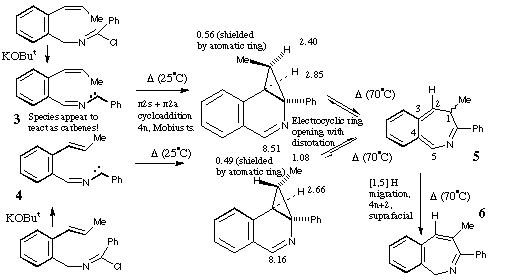
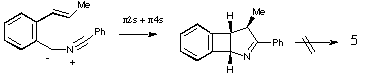
Qu 15. Species 3 and 4 appear to react as carbenes! The
two isomers X and Y are distinguished by the different chemical shift values of
the Me and H groups induced by the adjacent aromatic ring (anisotropy). The
observation that both X and Y can be isolated eliminates the possibility that
5 is formed directly from 3 or 4 by a 4n electron
electrocyclisation (assuming the inversion barrier in 5 is small!).
Another possibility of 3 or 4 acting as 1,3 dipoles and adding
directly to the alkene to give X or Y is eliminated by spectral evidence and
the necessity of the resulting cyclobutene having to ring open with disrotation
(Huckel ts) to give 5, a process not favoured by the selection rules.
See K R Motion, I. R. Robertson, J. T. Sharp and M. D. Walkinshaw, J. Chem,.
Soc., Perkin Trans 1, 1992, 1709 for further details.
Back to Problems
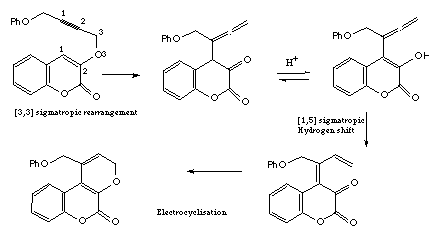
Qu 16N. Cairns, L. M. Harwood and D. P. Astles, J. Chem. Soc., Perkin
Trans 1, 1994, 3101, propose a so called "tandem" series of [3,3]
sigmatropic rearrangements, also known as Claisen rearrangements, for the
synthesis of a series of natural coumarins.

Back to Problems
Qu 17
P. W. Ambler, R. M. Paton and J. M. Trout, J. Chem. Soc., Chem.
Commun., 1994, 2661, report the use of norbornadiene as an acetylene
equivalent. The two adducts A and B are exo and
endo isomers, which are distinguished by different 3J
couplings, as predicted by the Karplus relationship. Molecular models show that
the vicinal angle in the exo isomer (~ 75*) is close to the
minimum in the Karplus function, whereas the endo isomer (~
40*) should exhibit larger couplings. The angle of ~ 0*
for the vicinal bridgehead protons leads to a medium sized coupling. The
ortho coupling in the isoxazole product is surprisingly small, although
it is in fact typical for such systems.

Back to Problems
Qu 18
Random dot autostereograms are restricted to representing images in a
series of planes. It is not possible to represent continuously varying depth.
The structure here is Gravelliferone (really!).
Back to Problems